 Found 952 hits with Last Name = 'yadav' and Initial = 'r'
Found 952 hits with Last Name = 'yadav' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50029257

((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...)Show SMILES CC(C)Oc1ccccc1N1CCN(Cc2cccc(c2)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C26H35N3O2/c1-21(2)31-25-12-5-4-11-24(25)28-17-15-27(16-18-28)20-22-9-8-10-23(19-22)26(30)29-13-6-3-7-14-29/h4-5,8-12,19,21H,3,6-7,13-18,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha1A receptor (unknown origin) |
Bioorg Med Chem 16: 4759-800 (2008)
Article DOI: 10.1016/j.bmc.2008.02.091
BindingDB Entry DOI: 10.7270/Q2DV1JPX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496138
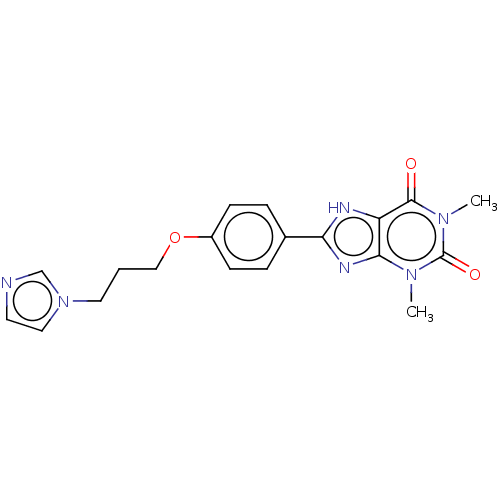
(CHEMBL3121727)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCn2ccnc2)cc1 Show InChI InChI=1S/C19H20N6O3/c1-23-17-15(18(26)24(2)19(23)27)21-16(22-17)13-4-6-14(7-5-13)28-11-3-9-25-10-8-20-12-25/h4-8,10,12H,3,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496133
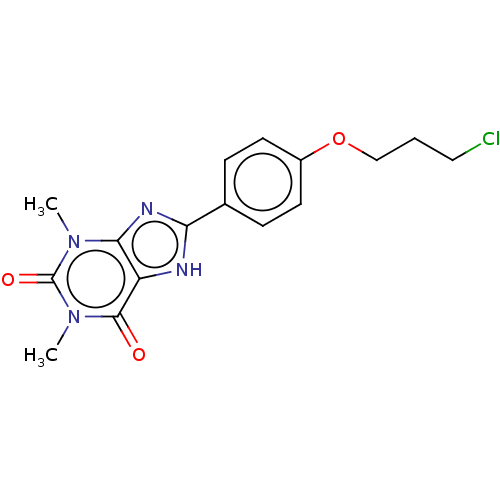
(CHEMBL3121725)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCCl)cc1 Show InChI InChI=1S/C16H17ClN4O3/c1-20-14-12(15(22)21(2)16(20)23)18-13(19-14)10-4-6-11(7-5-10)24-9-3-8-17/h4-7H,3,8-9H2,1-2H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496134
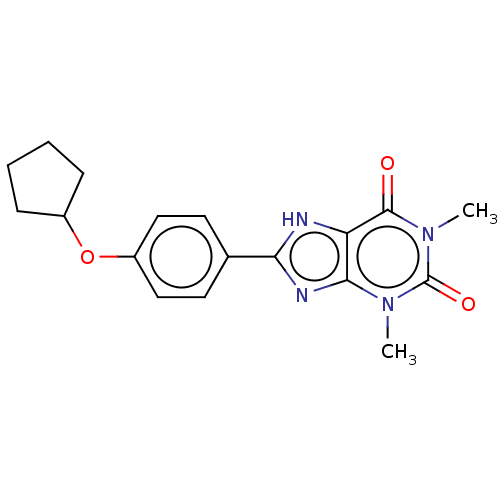
(CHEMBL3121726)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OC2CCCC2)cc1 Show InChI InChI=1S/C18H20N4O3/c1-21-16-14(17(23)22(2)18(21)24)19-15(20-16)11-7-9-13(10-8-11)25-12-5-3-4-6-12/h7-10,12H,3-6H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496142
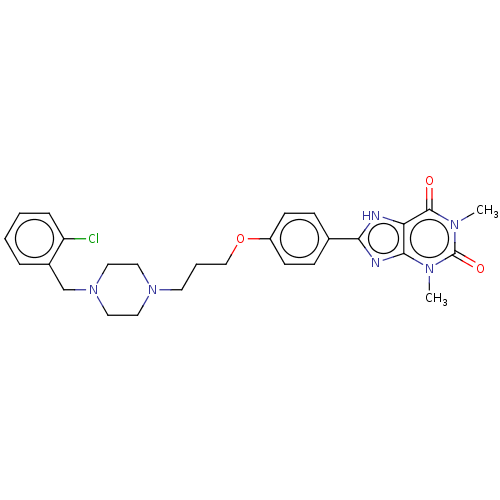
(CHEMBL3121728)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCN2CCN(Cc3ccccc3Cl)CC2)cc1 Show InChI InChI=1S/C27H31ClN6O3/c1-31-25-23(26(35)32(2)27(31)36)29-24(30-25)19-8-10-21(11-9-19)37-17-5-12-33-13-15-34(16-14-33)18-20-6-3-4-7-22(20)28/h3-4,6-11H,5,12-18H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12657
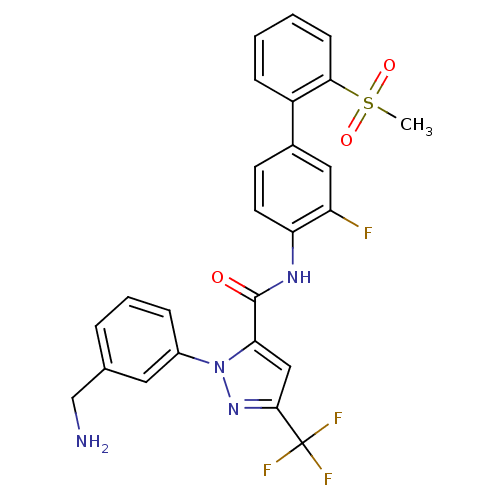
(1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H20F4N4O3S/c1-37(35,36)22-8-3-2-7-18(22)16-9-10-20(19(26)12-16)31-24(34)21-13-23(25(27,28)29)32-33(21)17-6-4-5-15(11-17)14-30/h2-13H,14,30H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50496142

(CHEMBL3121728)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCN2CCN(Cc3ccccc3Cl)CC2)cc1 Show InChI InChI=1S/C27H31ClN6O3/c1-31-25-23(26(35)32(2)27(31)36)29-24(30-25)19-8-10-21(11-9-19)37-17-5-12-33-13-15-34(16-14-33)18-20-6-3-4-7-22(20)28/h3-4,6-11H,5,12-18H2,1-2H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A2B receptor expressed in CHO cell membranes assessed as inhibition of NECA-stimulated adenylyl cycla... |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496131

(CHEMBL3121724)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C19H23N5O3/c1-22-17-15(18(25)23(2)19(22)26)20-16(21-17)13-5-7-14(8-6-13)27-12-11-24-9-3-4-10-24/h5-8H,3-4,9-12H2,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496141

(CHEMBL3121721)Show SMILES CCN(CC)CCOc1ccc(cc1)-c1nc2n(C)c(=O)n(C)c(=O)c2[nH]1 Show InChI InChI=1S/C19H25N5O3/c1-5-24(6-2)11-12-27-14-9-7-13(8-10-14)16-20-15-17(21-16)22(3)19(26)23(4)18(15)25/h7-10H,5-6,11-12H2,1-4H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496136

(CHEMBL3121722)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCC2CCNCC2)cc1 Show InChI InChI=1S/C20H25N5O3/c1-24-18-16(19(26)25(2)20(24)27)22-17(23-18)14-3-5-15(6-4-14)28-12-9-13-7-10-21-11-8-13/h3-6,13,21H,7-12H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496137
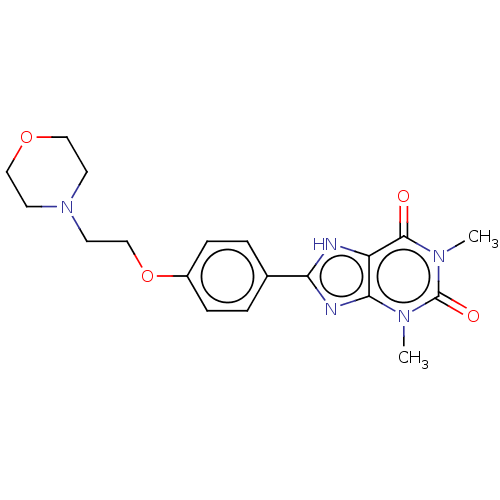
(CHEMBL3121723)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C19H23N5O4/c1-22-17-15(18(25)23(2)19(22)26)20-16(21-17)13-3-5-14(6-4-13)28-12-9-24-7-10-27-11-8-24/h3-6H,7-12H2,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50405890
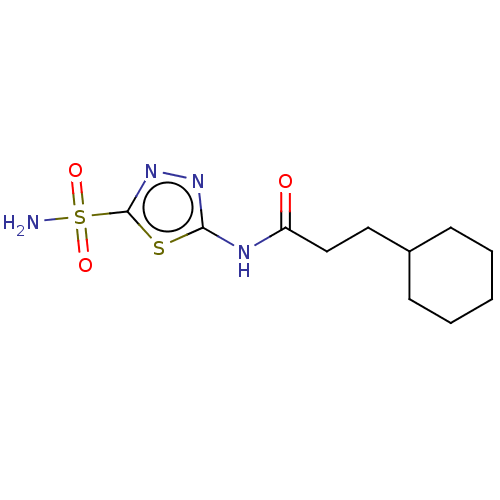
(CHEMBL4782535)Show InChI InChI=1S/C13H15NO5S2/c1-8(2)7-18-13(15)19-10-4-3-9-5-12(21(14,16)17)20-11(9)6-10/h3-6,8H,7H2,1-2H3,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50496135
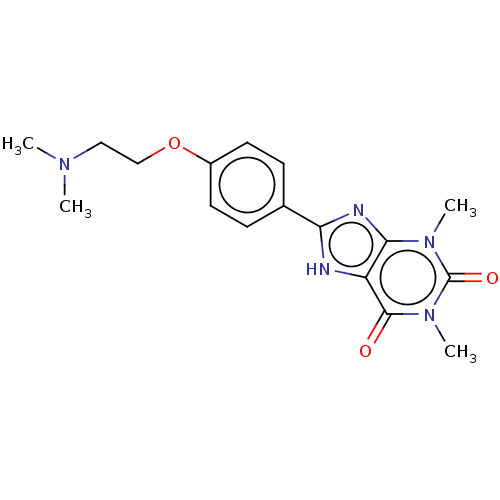
(CHEMBL3121720)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc2n(C)c(=O)n(C)c(=O)c2[nH]1 Show InChI InChI=1S/C17H21N5O3/c1-20(2)9-10-25-12-7-5-11(6-8-12)14-18-13-15(19-14)21(3)17(24)22(4)16(13)23/h5-8H,9-10H2,1-4H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NECA from human recombinant adenosine A2A receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614408
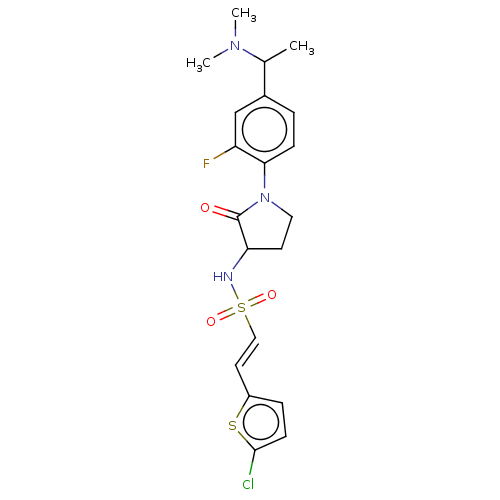
(CHEMBL5269594)Show SMILES CC(N(C)C)c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50496138

(CHEMBL3121727)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCn2ccnc2)cc1 Show InChI InChI=1S/C19H20N6O3/c1-23-17-15(18(26)24(2)19(23)27)21-16(22-17)13-4-6-14(7-5-13)28-11-3-9-25-10-8-20-12-25/h4-8,10,12H,3,9,11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096104

(2-(3-Aminomethyl-phenyl)-5-trifluoromethyl-2H-pyra...)Show SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F4N5O3S/c25-18-11-15(17-6-1-2-7-21(17)37(30,35)36)8-9-19(18)31-23(34)20-12-22(24(26,27)28)32-33(20)16-5-3-4-14(10-16)13-29/h1-12H,13,29H2,(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50004154

(2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(O)=O |c:7,t:4| Show InChI InChI=1S/C24H25ClN6O2/c1-3-4-9-20-26-22(25)21(24(32)33)15(2)31(20)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)23-27-29-30-28-23/h5-8,10-13,15H,3-4,9,14H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136071
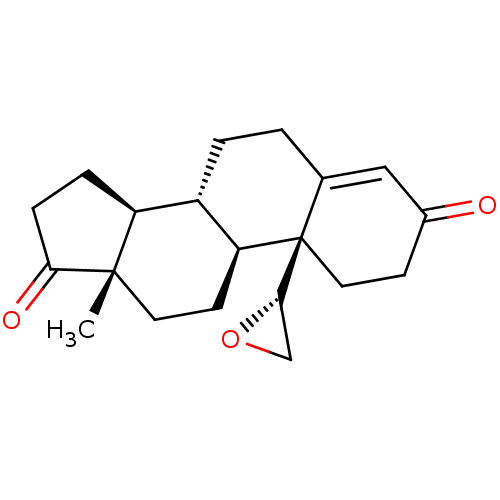
(CHEMBL3753593)Show SMILES [H][C@]1(CO1)[C@]12CCC(=O)C=C1CC[C@@]1([H])[C@]3([H])CCC(=O)[C@@]3(C)CC[C@]21[H] |r,c:10| Show InChI InChI=1S/C20H26O3/c1-19-8-7-16-14(15(19)4-5-17(19)22)3-2-12-10-13(21)6-9-20(12,16)18-11-23-18/h10,14-16,18H,2-9,11H2,1H3/t14-,15-,16-,18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50496134

(CHEMBL3121726)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OC2CCCC2)cc1 Show InChI InChI=1S/C18H20N4O3/c1-21-16-14(17(23)22(2)18(21)24)19-15(20-16)11-7-9-13(10-8-11)25-12-5-3-4-6-12/h7-10,12H,3-6H2,1-2H3,(H,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A2B receptor expressed in CHO cell membranes assessed as inhibition of NECA-stimulated adenylyl cycla... |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614410
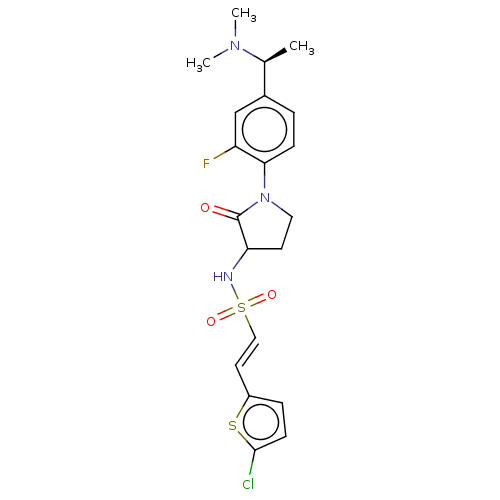
(CHEMBL5286872)Show SMILES C[C@H](N(C)C)c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50496138

(CHEMBL3121727)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCn2ccnc2)cc1 Show InChI InChI=1S/C19H20N6O3/c1-23-17-15(18(26)24(2)19(23)27)21-16(22-17)13-4-6-14(7-5-13)28-11-3-9-25-10-8-20-12-25/h4-8,10,12H,3,9,11H2,1-2H3,(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A2B receptor expressed in CHO cell membranes assessed as inhibition of NECA-stimulated adenylyl cycla... |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50496131

(CHEMBL3121724)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C19H23N5O3/c1-22-17-15(18(25)23(2)19(22)26)20-16(21-17)13-5-7-14(8-6-13)27-12-11-24-9-3-4-10-24/h5-8H,3-4,9-12H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50496136

(CHEMBL3121722)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCC2CCNCC2)cc1 Show InChI InChI=1S/C20H25N5O3/c1-24-18-16(19(26)25(2)20(24)27)22-17(23-18)14-3-5-15(6-4-14)28-12-9-13-7-10-21-11-8-13/h3-6,13,21H,7-12H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50496137

(CHEMBL3121723)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C19H23N5O4/c1-22-17-15(18(25)23(2)19(22)26)20-16(21-17)13-3-5-14(6-4-13)28-12-9-24-7-10-27-11-8-24/h3-6H,7-12H2,1-2H3,(H,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A2B receptor expressed in CHO cell membranes assessed as inhibition of NECA-stimulated adenylyl cycla... |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135997

(CHEMBL3754471)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](C)C2=CC(=O)CC[C@]12C |r,t:19| Show InChI InChI=1S/C20H28O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11-12,14-16H,4-10H2,1-3H3/t12-,14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136063

(CHEMBL3752102)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CCCC)C2=CC(=O)CC[C@]12C |r,t:22| Show InChI InChI=1S/C23H34O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h14-15,17-19H,4-13H2,1-3H3/t15-,17+,18+,19+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136057
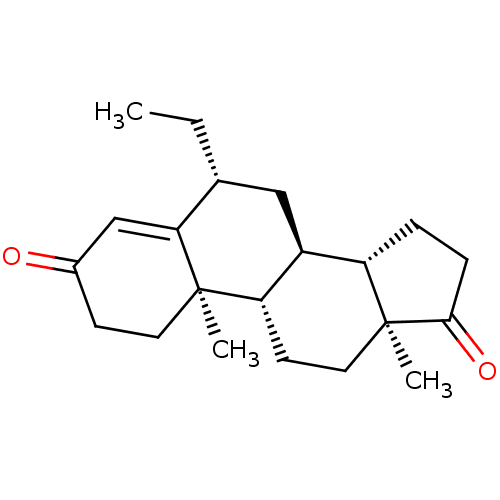
(CHEMBL3752661)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CC)C2=CC(=O)CC[C@]12C |r,t:20| Show InChI InChI=1S/C21H30O2/c1-4-13-11-15-16-5-6-19(23)21(16,3)10-8-17(15)20(2)9-7-14(22)12-18(13)20/h12-13,15-17H,4-11H2,1-3H3/t13-,15+,16+,17+,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136000
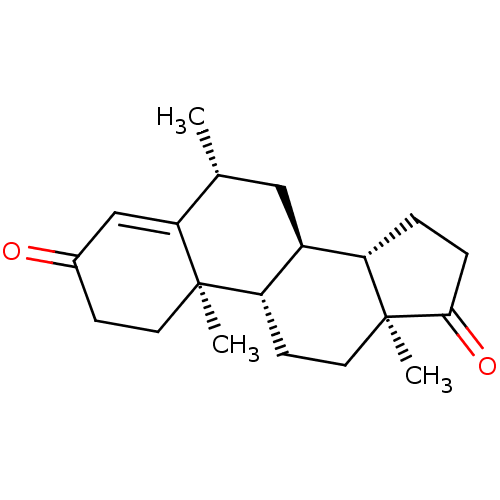
(CHEMBL3754220)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](C)C2=CC(=O)CC[C@]12C |r,t:19| Show InChI InChI=1S/C20H28O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11-12,14-16H,4-10H2,1-3H3/t12-,14+,15+,16+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135998

(CHEMBL3752668)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](CCCC)C2=CC(=O)CC[C@]12C |r,t:22| Show InChI InChI=1S/C23H34O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h14-15,17-19H,4-13H2,1-3H3/t15-,17-,18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50496141

(CHEMBL3121721)Show SMILES CCN(CC)CCOc1ccc(cc1)-c1nc2n(C)c(=O)n(C)c(=O)c2[nH]1 Show InChI InChI=1S/C19H25N5O3/c1-5-24(6-2)11-12-27-14-9-7-13(8-10-14)16-20-15-17(21-16)22(3)19(26)23(4)18(15)25/h7-10H,5-6,11-12H2,1-4H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50506000

(CHEMBL4445337)Show SMILES N[C@@H](CCN(CC#Cc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H28N8O6/c25-15(24(36)37)6-8-31(7-2-4-13-3-1-5-14(9-13)21(27)35)10-16-18(33)19(34)23(38-16)32-12-30-17-20(26)28-11-29-22(17)32/h1,3,5,9,11-12,15-16,18-19,23,33-34H,6-8,10,25H2,(H2,27,35)(H,36,37)(H2,26,28,29)/t15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal TEV cleavage site NNMT (1 to 270 residues) expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL cells us... |
J Med Chem 62: 10783-10797 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01255
BindingDB Entry DOI: 10.7270/Q2WD43VS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030727
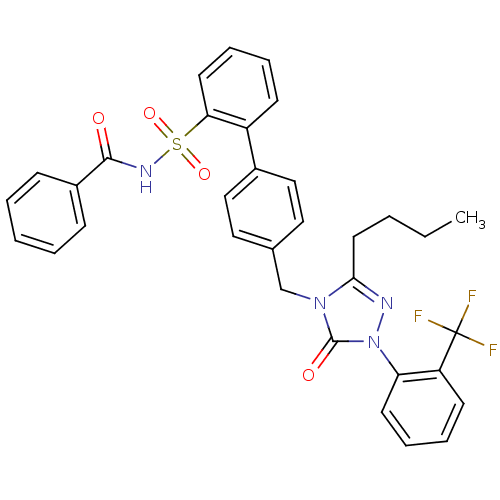
(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT1 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50496135

(CHEMBL3121720)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc2n(C)c(=O)n(C)c(=O)c2[nH]1 Show InChI InChI=1S/C17H21N5O3/c1-20(2)9-10-25-12-7-5-11(6-8-12)14-18-13-15(19-14)21(3)17(24)22(4)16(13)23/h5-8H,9-10H2,1-4H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50496142

(CHEMBL3121728)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCN2CCN(Cc3ccccc3Cl)CC2)cc1 Show InChI InChI=1S/C27H31ClN6O3/c1-31-25-23(26(35)32(2)27(31)36)29-24(30-25)19-8-10-21(11-9-19)37-17-5-12-33-13-15-34(16-14-33)18-20-6-3-4-7-22(20)28/h3-4,6-11H,5,12-18H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50506000

(CHEMBL4445337)Show SMILES N[C@@H](CCN(CC#Cc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H28N8O6/c25-15(24(36)37)6-8-31(7-2-4-13-3-1-5-14(9-13)21(27)35)10-16-18(33)19(34)23(38-16)32-12-30-17-20(26)28-11-29-22(17)32/h1,3,5,9,11-12,15-16,18-19,23,33-34H,6-8,10,25H2,(H2,27,35)(H,36,37)(H2,26,28,29)/t15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal TEV cleavage site NNMT (1 to 270 residues) expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL cells us... |
J Med Chem 62: 10783-10797 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01255
BindingDB Entry DOI: 10.7270/Q2WD43VS |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50506000

(CHEMBL4445337)Show SMILES N[C@@H](CCN(CC#Cc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H28N8O6/c25-15(24(36)37)6-8-31(7-2-4-13-3-1-5-14(9-13)21(27)35)10-16-18(33)19(34)23(38-16)32-12-30-17-20(26)28-11-29-22(17)32/h1,3,5,9,11-12,15-16,18-19,23,33-34H,6-8,10,25H2,(H2,27,35)(H,36,37)(H2,26,28,29)/t15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal TEV cleavage site NNMT (1 to 270 residues) expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL cells us... |
J Med Chem 62: 10783-10797 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01255
BindingDB Entry DOI: 10.7270/Q2WD43VS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50270453
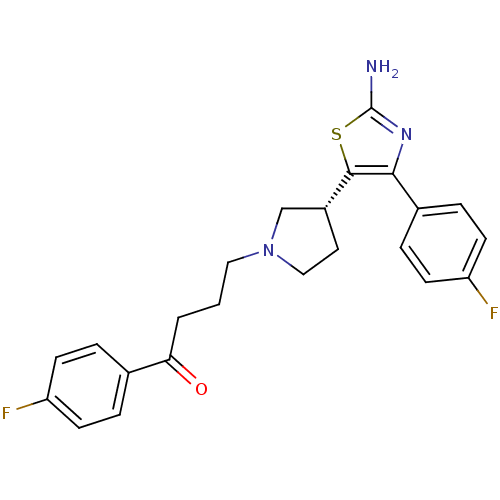
((R)-4-(3-(2-amino-4-(4-fluorophenyl)thiazol-5-yl)p...)Show SMILES Nc1nc(c(s1)[C@@H]1CCN(CCCC(=O)c2ccc(F)cc2)C1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H23F2N3OS/c24-18-7-3-15(4-8-18)20(29)2-1-12-28-13-11-17(14-28)22-21(27-23(26)30-22)16-5-9-19(25)10-6-16/h3-10,17H,1-2,11-14H2,(H2,26,27)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A (unknown origin) |
Bioorg Med Chem 16: 4759-800 (2008)
Article DOI: 10.1016/j.bmc.2008.02.091
BindingDB Entry DOI: 10.7270/Q2DV1JPX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50496131

(CHEMBL3121724)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C19H23N5O3/c1-22-17-15(18(25)23(2)19(22)26)20-16(21-17)13-5-7-14(8-6-13)27-12-11-24-9-3-4-10-24/h5-8H,3-4,9-12H2,1-2H3,(H,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A2B receptor expressed in CHO cell membranes assessed as inhibition of NECA-stimulated adenylyl cycla... |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614409
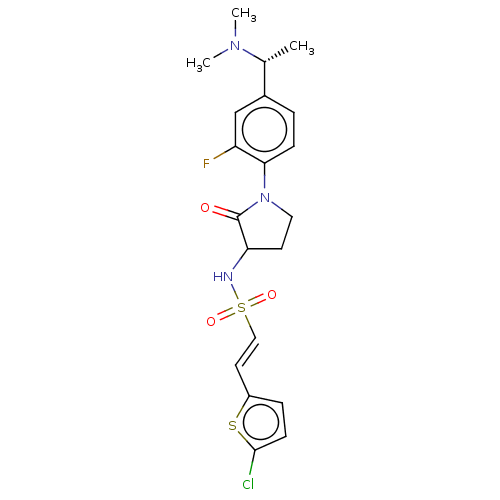
(CHEMBL5276446)Show SMILES C[C@@H](N(C)C)c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614403
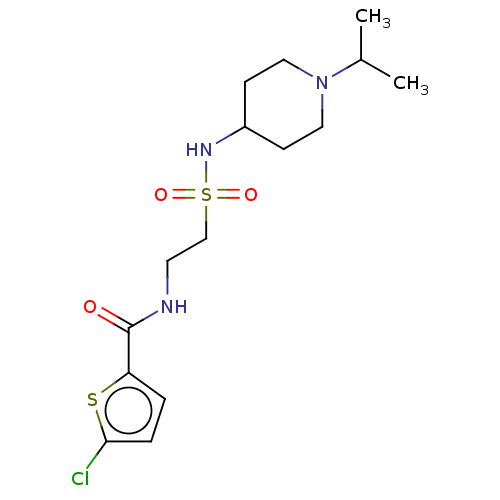
(CHEMBL5291366)Show SMILES CC(C)N1CCC(CC1)NS(=O)(=O)CCNC(=O)c1ccc(Cl)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614404
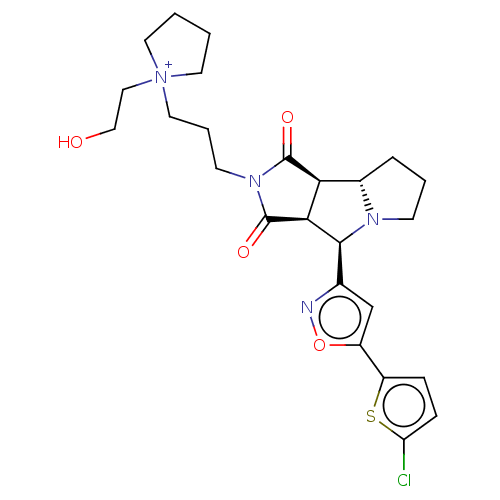
(CHEMBL5266057)Show SMILES [H][C@@]12CCCN1[C@@H](c1cc(on1)-c1ccc(Cl)s1)[C@@]1([H])C(=O)N(CCC[N+]3(CCO)CCCC3)C(=O)[C@@]21[H] |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50496138

(CHEMBL3121727)Show SMILES Cn1c2nc([nH]c2c(=O)n(C)c1=O)-c1ccc(OCCCn2ccnc2)cc1 Show InChI InChI=1S/C19H20N6O3/c1-23-17-15(18(26)24(2)19(23)27)21-16(22-17)13-4-6-14(7-5-13)28-11-3-9-25-10-8-20-12-25/h4-8,10,12H,3,9,11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-HEMADO from human recombinant adenosine A3 receptor expressed in CHO cell membranes after 3 hrs by microbeta counting analysis |
Eur J Med Chem 75: 327-35 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.045
BindingDB Entry DOI: 10.7270/Q20R9SC2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 17: 444-59 (2009)
Article DOI: 10.1016/j.bmc.2008.11.067
BindingDB Entry DOI: 10.7270/Q2WH2QWZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135970
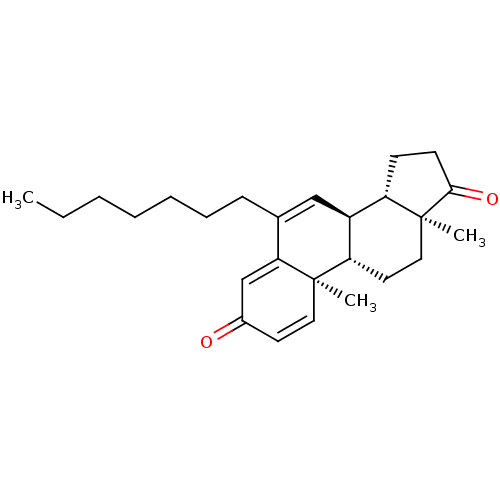
(CHEMBL3752341)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCCCCC)C2=CC(=O)C=C[C@]12C |r,c:29,t:16,25| Show InChI InChI=1S/C26H36O2/c1-4-5-6-7-8-9-18-16-20-21-10-11-24(28)26(21,3)15-13-22(20)25(2)14-12-19(27)17-23(18)25/h12,14,16-17,20-22H,4-11,13,15H2,1-3H3/t20-,21-,22-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50421878
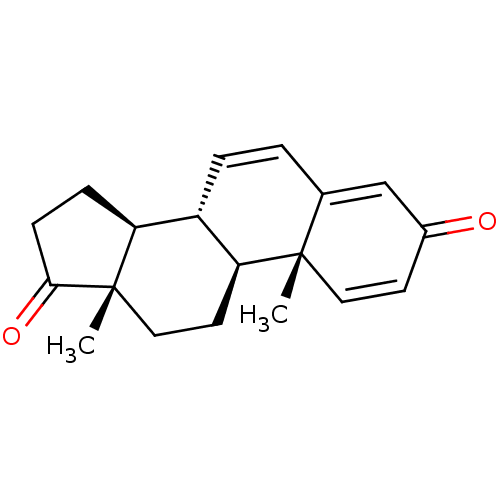
(CHEMBL2311178)Show SMILES C[C@]12CC[C@H]3[C@@H](C=CC4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |c:6,12,t:8| Show InChI InChI=1S/C19H22O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3-4,7,9,11,14-16H,5-6,8,10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135969

(CHEMBL3752315)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCCCC)C2=CC(=O)C=C[C@]12C |r,c:28,t:16,24| Show InChI InChI=1S/C25H34O2/c1-4-5-6-7-8-17-15-19-20-9-10-23(27)25(20,3)14-12-21(19)24(2)13-11-18(26)16-22(17)24/h11,13,15-16,19-21H,4-10,12,14H2,1-3H3/t19-,20-,21-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135922
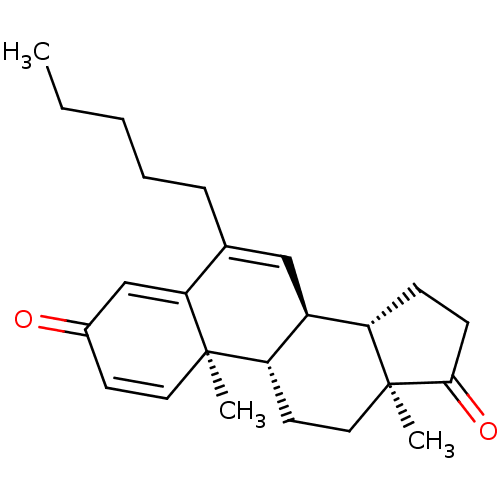
(CHEMBL3752619)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCCC)C2=CC(=O)C=C[C@]12C |r,c:27,t:16,23| Show InChI InChI=1S/C24H32O2/c1-4-5-6-7-16-14-18-19-8-9-22(26)24(19,3)13-11-20(18)23(2)12-10-17(25)15-21(16)23/h10,12,14-15,18-20H,4-9,11,13H2,1-3H3/t18-,19-,20-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135921

(CHEMBL3752011)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCC)C2=CC(=O)C=C[C@]12C |r,c:26,t:16,22| Show InChI InChI=1S/C23H30O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h9,11,13-14,17-19H,4-8,10,12H2,1-3H3/t17-,18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135918
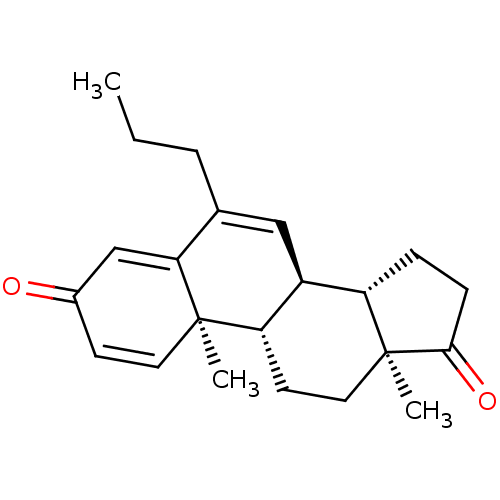
(CHEMBL3753803)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCC)C2=CC(=O)C=C[C@]12C |r,c:25,t:16,21| Show InChI InChI=1S/C22H28O2/c1-4-5-14-12-16-17-6-7-20(24)22(17,3)11-9-18(16)21(2)10-8-15(23)13-19(14)21/h8,10,12-13,16-18H,4-7,9,11H2,1-3H3/t16-,17-,18-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data