

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50010070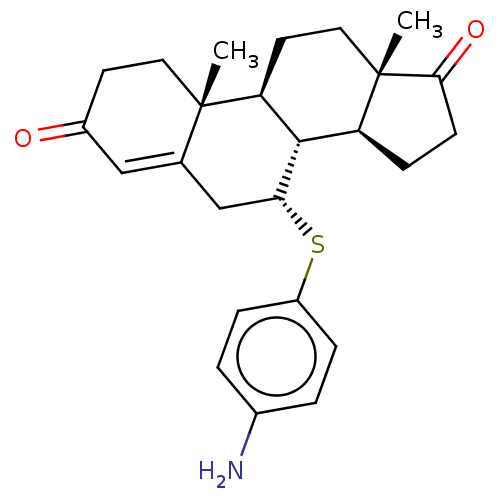 (CHEMBL3245357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010069 (CHEMBL3245356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010067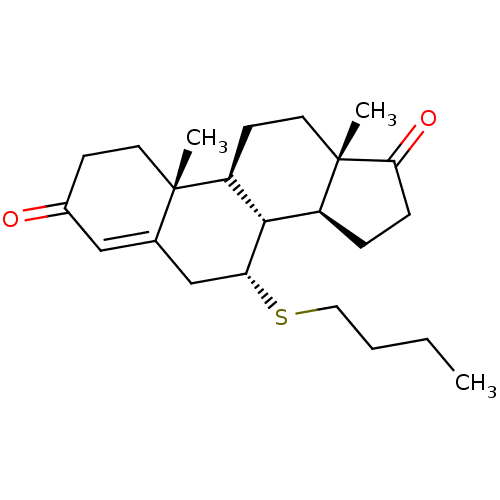 (CHEMBL3245348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010068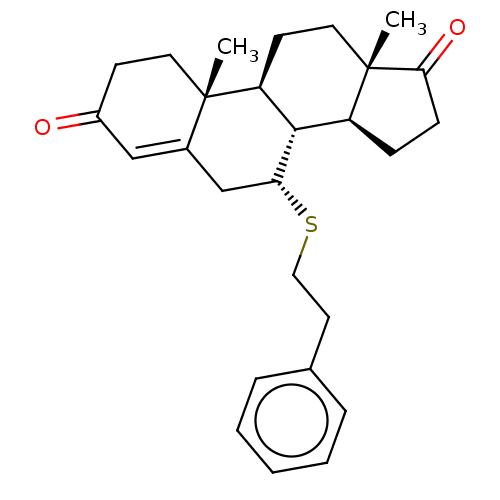 (CHEMBL3245353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010071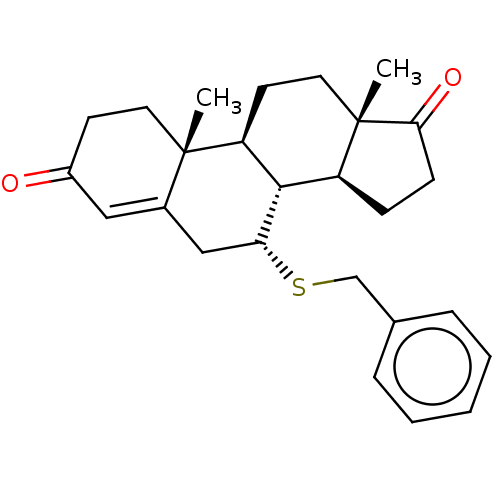 (CHEMBL3245350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Bos taurus) | BDBM50028166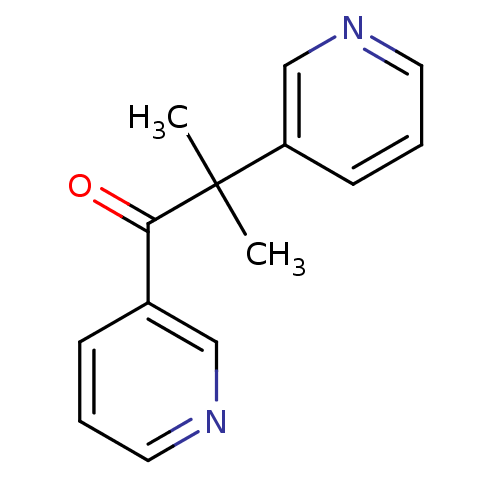 (CHEMBL934 | METYRAPONE | US9138393, Metyrapone | U...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of bovine adrenal gland 11beta-hydroxylase assessed as inhibition of [14C]-deoxycorticosterone hydroxylation | J Med Chem 20: 762-6 (1977) BindingDB Entry DOI: 10.7270/Q2639R92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50021809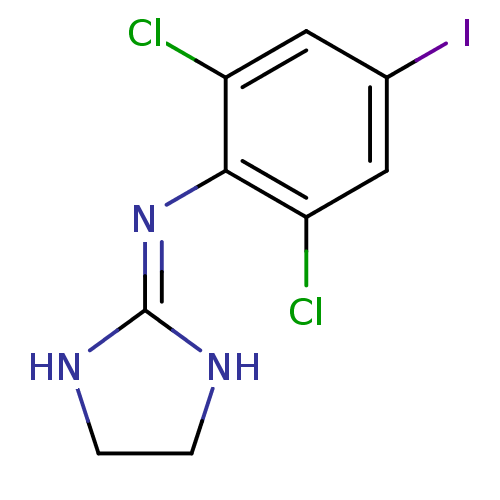 ((2,6-Dichloro-4-iodo-phenyl)-(4,5-dihydro-1H-imida...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM34572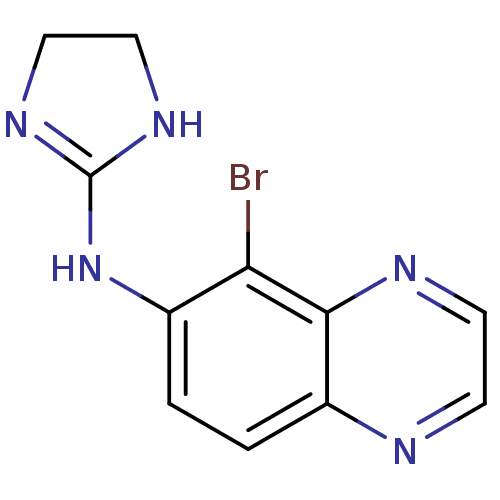 (BRIMONIDINE | CHEMBL844 | MLS000069370 | SMR000058...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50021812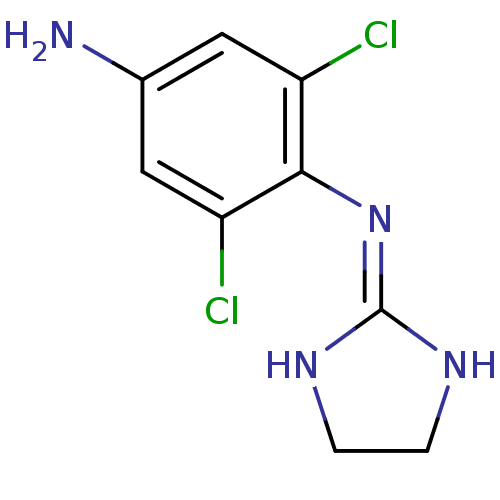 (2,6-Dichloro-N-imidazolidin-2-ylidene-benzene-1,4-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50029050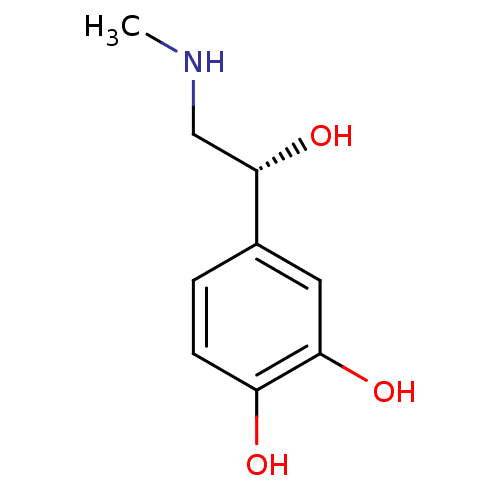 ((-)-(R)-epinephrine | (-)-3,4-dihydroxy-alpha-((me...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50225285 (CHEBI:3757 | Catapres | Catapres-Tts-1 | Catapres-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50226861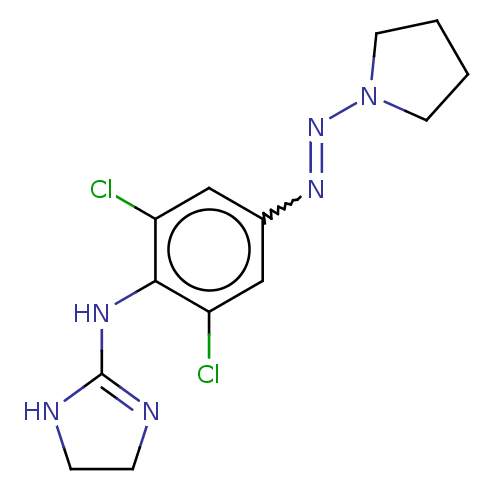 (CHEMBL22073) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50226860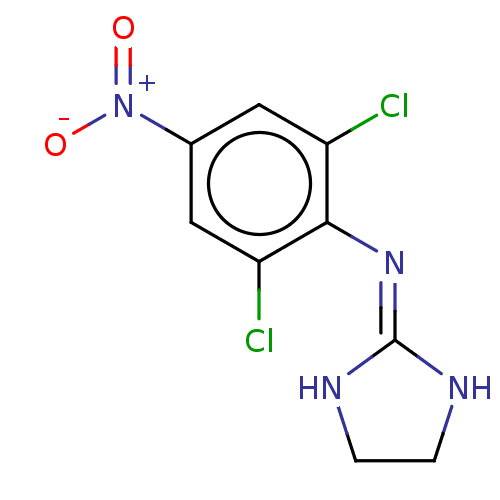 (CHEMBL21734) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranes | J Med Chem 30: 1241-4 (1987) BindingDB Entry DOI: 10.7270/Q2JM2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||