 Found 34 hits with Last Name = 'akber ansari' and Initial = 's'
Found 34 hits with Last Name = 'akber ansari' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50513900

(CHEMBL4444582)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)n1[nH]c(=O)c2cc(F)ccc2c1=O Show InChI InChI=1S/C15H10FN3O5S/c16-9-3-6-11-12(7-9)13(20)18-19(15(11)22)14(21)8-1-4-10(5-2-8)25(17,23)24/h1-7H,(H,18,20)(H2,17,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50513907

(CHEMBL4579543)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)n1[nH]c(=O)c2c(F)cccc2c1=O Show InChI InChI=1S/C15H10FN3O5S/c16-11-3-1-2-10-12(11)13(20)18-19(15(10)22)14(21)8-4-6-9(7-5-8)25(17,23)24/h1-7H,(H,18,20)(H2,17,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50513912
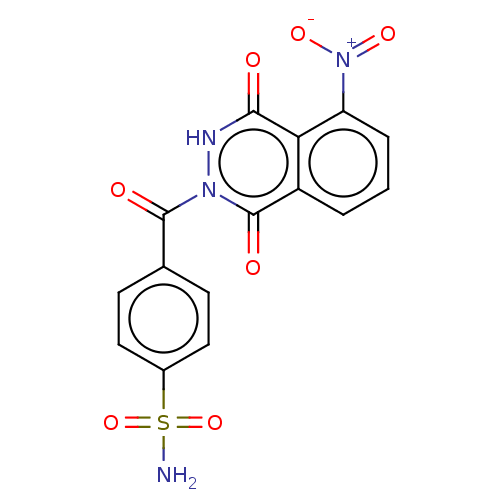
(CHEMBL4524569)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)n1[nH]c(=O)c2c(cccc2c1=O)[N+]([O-])=O Show InChI InChI=1S/C15H10N4O7S/c16-27(25,26)9-6-4-8(5-7-9)14(21)18-15(22)10-2-1-3-11(19(23)24)12(10)13(20)17-18/h1-7H,(H,17,20)(H2,16,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50513908

(CHEMBL4453373)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)n1[nH]c(=O)c2cc(ccc2c1=O)[N+]([O-])=O Show InChI InChI=1S/C15H10N4O7S/c16-27(25,26)10-4-1-8(2-5-10)14(21)18-15(22)11-6-3-9(19(23)24)7-12(11)13(20)17-18/h1-7H,(H,17,20)(H2,16,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50513909

(CHEMBL4541764)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)n1[nH]c(=O)c2ccc(cc2c1=O)C(O)=O Show InChI InChI=1S/C16H11N3O7S/c17-27(25,26)10-4-1-8(2-5-10)14(21)19-15(22)12-7-9(16(23)24)3-6-11(12)13(20)18-19/h1-7H,(H,18,20)(H,23,24)(H2,17,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50513904
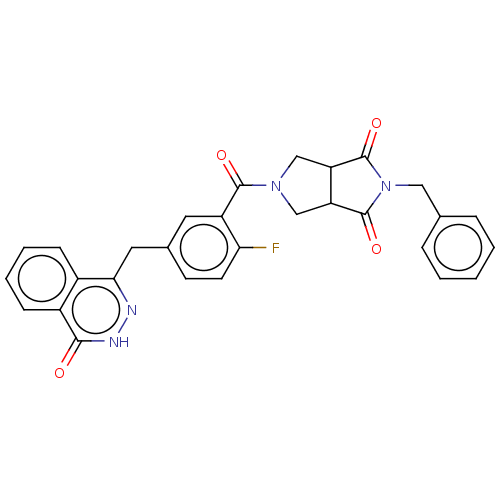
(CHEMBL4546114)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CC2C(C1)C(=O)N(Cc1ccccc1)C2=O Show InChI InChI=1S/C29H23FN4O4/c30-24-11-10-18(13-25-19-8-4-5-9-20(19)26(35)32-31-25)12-21(24)27(36)33-15-22-23(16-33)29(38)34(28(22)37)14-17-6-2-1-3-7-17/h1-12,22-23H,13-16H2,(H,32,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 expressed in Escherichia coli incubated for 15 to 40 mins by ELISA |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50513902

(CHEMBL4442022)Show SMILES FC(F)(F)c1nnc2CN(CCn12)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)cc2CCOc12 Show InChI InChI=1S/C24H19F3N6O3/c25-24(26,27)23-31-29-19-12-32(6-7-33(19)23)22(35)17-10-13(9-14-5-8-36-20(14)17)11-18-15-3-1-2-4-16(15)21(34)30-28-18/h1-4,9-10H,5-8,11-12H2,(H,30,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 expressed in Escherichia coli incubated for 15 to 40 mins by ELISA |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50513901

(CHEMBL4579526)Show SMILES CCN1CCCC2CN(CC12)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C25H27FN4O2/c1-2-29-11-5-6-17-14-30(15-23(17)29)25(32)20-12-16(9-10-21(20)26)13-22-18-7-3-4-8-19(18)24(31)28-27-22/h3-4,7-10,12,17,23H,2,5-6,11,13-15H2,1H3,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 expressed in Escherichia coli using histone as substrate by ELISA |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human placenta Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50006469

(CHEMBL69956 | [8-Oxo-7-(5-trifluoromethyl-benzothi...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ncccc12 Show InChI InChI=1S/C18H11F3N4O3S/c19-18(20,21)9-3-4-13-12(6-9)23-14(29-13)8-25-17(28)16-10(2-1-5-22-16)11(24-25)7-15(26)27/h1-6H,7-8H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50268124

(CHEMBL4078014)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCN(CC2)C(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)cc1 Show InChI InChI=1S/C30H28FN5O4/c31-26-11-9-22(18-27-23-3-1-2-4-24(23)29(38)33-32-27)17-25(26)30(39)36-15-13-35(14-16-36)19-21-7-5-20(6-8-21)10-12-28(37)34-40/h1-12,17,40H,13-16,18-19H2,(H,33,38)(H,34,37)/b12-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50009781
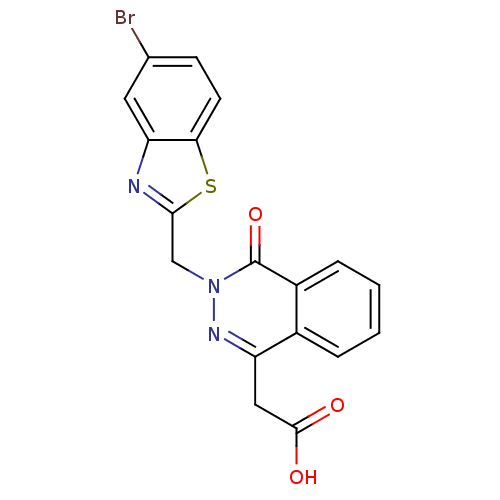
(CHEMBL20169 | [3-(5-Bromo-benzothiazol-2-ylmethyl)...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(Br)ccc3s2)c(=O)c2ccccc12 Show InChI InChI=1S/C18H12BrN3O3S/c19-10-5-6-15-14(7-10)20-16(26-15)9-22-18(25)12-4-2-1-3-11(12)13(21-22)8-17(23)24/h1-7H,8-9H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human placenta Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50008471

(CHEMBL143234 | [3-(5,7-Difluoro-benzooxazol-2-ylme...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(F)cc(F)c3o2)c(=O)c2ccccc12 Show InChI InChI=1S/C18H11F2N3O4/c19-9-5-12(20)17-14(6-9)21-15(27-17)8-23-18(26)11-4-2-1-3-10(11)13(22-23)7-16(24)25/h1-6H,7-8H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human placenta Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50006481

(CHEMBL73560 | [1-(5,7-Difluoro-benzothiazol-2-ylme...)Show SMILES Cc1c(CC(O)=O)nn(Cc2nc3cc(F)cc(F)c3s2)c(=O)c1C Show InChI InChI=1S/C16H13F2N3O3S/c1-7-8(2)16(24)21(20-11(7)5-14(22)23)6-13-19-12-4-9(17)3-10(18)15(12)25-13/h3-4H,5-6H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) by colorimetric assay |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50006471
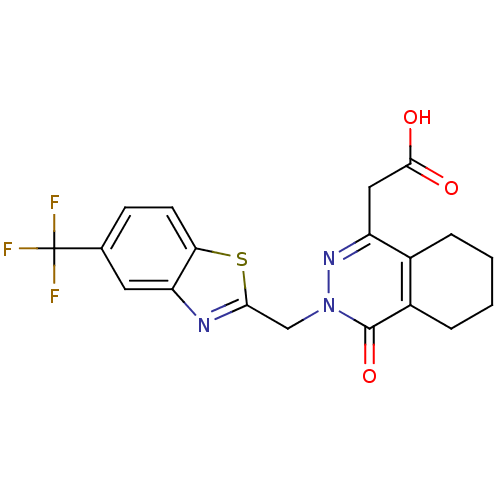
(CHEMBL68580 | [4-Oxo-3-(5-trifluoromethyl-benzothi...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2CCCCc12 Show InChI InChI=1S/C19H16F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h5-7H,1-4,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50268134

(CHEMBL4097093)Show SMILES ONC(=O)c1ccc(CN2CCN(CC2)C(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)cc1 Show InChI InChI=1S/C28H26FN5O4/c29-24-10-7-19(16-25-21-3-1-2-4-22(21)27(36)31-30-25)15-23(24)28(37)34-13-11-33(12-14-34)17-18-5-8-20(9-6-18)26(35)32-38/h1-10,15,38H,11-14,16-17H2,(H,31,36)(H,32,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) by colorimetric assay |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 expressed in Escherichia coli incubated for 10 mins by colorimetric assay |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50513903

(CHEMBL4547590)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCn2c(C1)nnc2C1CC1 Show InChI InChI=1S/C24H21FN6O2/c25-19-8-5-14(12-20-16-3-1-2-4-17(16)23(32)29-26-20)11-18(19)24(33)30-9-10-31-21(13-30)27-28-22(31)15-6-7-15/h1-5,8,11,15H,6-7,9-10,12-13H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 expressed in Escherichia coli incubated for 10 mins by colorimetric assay |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50006463

((3-Benzothiazol-2-ylmethyl-4-oxo-3,4-dihydro-phtha...)Show InChI InChI=1S/C18H13N3O3S/c22-17(23)9-14-11-5-1-2-6-12(11)18(24)21(20-14)10-16-19-13-7-3-4-8-15(13)25-16/h1-8H,9-10H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human placenta Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50513914
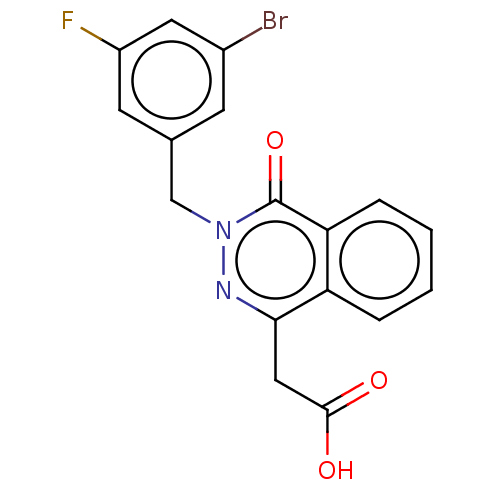
(CHEMBL4436968)Show SMILES OC(=O)Cc1nn(Cc2cc(F)cc(Br)c2)c(=O)c2ccccc12 Show InChI InChI=1S/C17H12BrFN2O3/c18-11-5-10(6-12(19)7-11)9-21-17(24)14-4-2-1-3-13(14)15(20-21)8-16(22)23/h1-7H,8-9H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50513913
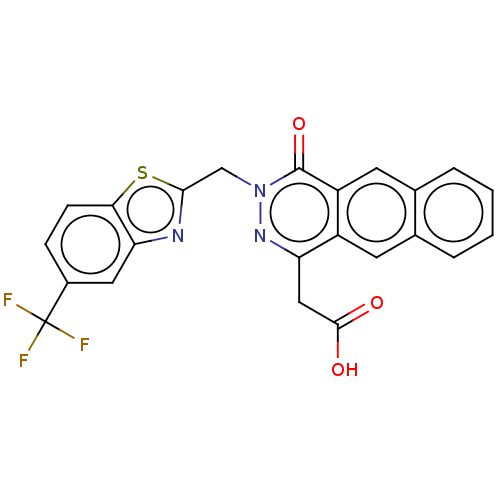
(CHEMBL4458061)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2cc3ccccc3cc12 Show InChI InChI=1S/C23H14F3N3O3S/c24-23(25,26)14-5-6-19-18(9-14)27-20(33-19)11-29-22(32)16-8-13-4-2-1-3-12(13)7-15(16)17(28-29)10-21(30)31/h1-9H,10-11H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human Aldose reductase |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50028859
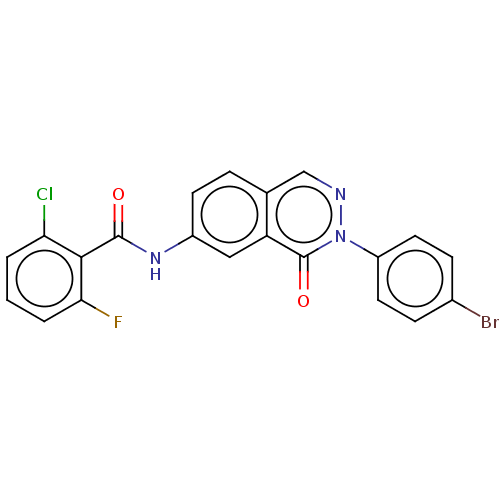
(CHEMBL3342698)Show SMILES Fc1cccc(Cl)c1C(=O)Nc1ccc2cnn(-c3ccc(Br)cc3)c(=O)c2c1 Show InChI InChI=1S/C21H12BrClFN3O2/c22-13-5-8-15(9-6-13)27-21(29)16-10-14(7-4-12(16)11-25-27)26-20(28)19-17(23)2-1-3-18(19)24/h1-11H,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells assessed as reduction in IL-beta induced PGE2 release preincubated for 30 mins followed by IL-beta addition... |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50513910

(CHEMBL4450818)Show SMILES Clc1cccc(NC(=O)CCCn2nc(-c3ccccc3)c3ccccc3c2=O)c1 Show InChI InChI=1S/C24H20ClN3O2/c25-18-10-6-11-19(16-18)26-22(29)14-7-15-28-24(30)21-13-5-4-12-20(21)23(27-28)17-8-2-1-3-9-17/h1-6,8-13,16H,7,14-15H2,(H,26,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human PARP1 expressed in a Baculovirus infected Sf9 insect cells using biotinylated substrate incubated for 1 hr ... |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4851
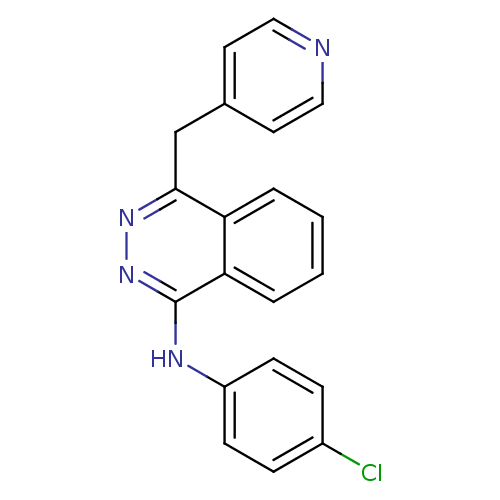
((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...)Show InChI InChI=1S/C20H15ClN4/c21-15-5-7-16(8-6-15)23-20-18-4-2-1-3-17(18)19(24-25-20)13-14-9-11-22-12-10-14/h1-12H,13H2,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 (unknown origin) |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4851

((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...)Show InChI InChI=1S/C20H15ClN4/c21-15-5-7-16(8-6-15)23-20-18-4-2-1-3-17(18)19(24-25-20)13-14-9-11-22-12-10-14/h1-12H,13H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human PARP1 expressed in a Baculovirus infected Sf9 insect cells using biotinylated substrate incubated for 1 hr ... |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50513905

(CHEMBL4475847)Show SMILES CC(C)n1nc(Nc2cc(C)[nH]n2)c2ccc(OCCN3CCOCC3)cc2c1=O Show InChI InChI=1S/C21H28N6O3/c1-14(2)27-21(28)18-13-16(30-11-8-26-6-9-29-10-7-26)4-5-17(18)20(25-27)22-19-12-15(3)23-24-19/h4-5,12-14H,6-11H2,1-3H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His4-tagged Aurora A (unknown origin) using PKB-GSK2 biotinylated peptide as substrate incubated for 90 mins by ELISA |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50513911

(CHEMBL4580842)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCn2cnnc2C1 Show InChI InChI=1S/C21H17FN6O2/c22-17-6-5-13(10-18-14-3-1-2-4-15(14)20(29)26-24-18)9-16(17)21(30)27-7-8-28-12-23-25-19(28)11-27/h1-6,9,12H,7-8,10-11H2,(H,26,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 expressed in Escherichia coli incubated for 15 to 40 mins by ELISA |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 1
(Ovis aries) | BDBM50513906

(CHEMBL4549769)Show SMILES Cc1ccc(cc1C)-c1nn(-c2ccc(cc2)S(N)(=O)=O)c(=O)c2ccccc12 Show InChI InChI=1S/C22H19N3O3S/c1-14-7-8-16(13-15(14)2)21-19-5-3-4-6-20(19)22(26)25(24-21)17-9-11-18(12-10-17)29(23,27)28/h3-13H,1-2H3,(H2,23,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 preincubated for 5 mins followed by arachidonic acid addition and measured after 2 mins by colorimetric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 1
(Ovis aries) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 preincubated for 5 mins followed by arachidonic acid addition and measured after 2 mins by colorimetric method |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Homo sapiens (Human)) | BDBM160509

(US10280160, Example 1. | US9682969, 1)Show SMILES CN(C(=O)Cn1nc(-c2cccc(Cl)c2)c2ccccc2c1=O)c1ccc2nc(C)oc2c1 Show InChI InChI=1S/C25H19ClN4O3/c1-15-27-21-11-10-18(13-22(21)33-15)29(2)23(31)14-30-25(32)20-9-4-3-8-19(20)24(28-30)16-6-5-7-17(26)12-16/h3-13H,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a |
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human CFTR F508 deletion mutant expressed in rat FRT cells coexpressing YFP-H148Q/I152L 25,22 incubated for 60 to 120 mins in presence ... |
Bioorg Med Chem 27: 3979-3997 (2019)
Article DOI: 10.1016/j.bmc.2019.07.050
BindingDB Entry DOI: 10.7270/Q28W3HMG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data