 Found 4286 hits with Last Name = 'bach' and Initial = 's'
Found 4286 hits with Last Name = 'bach' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
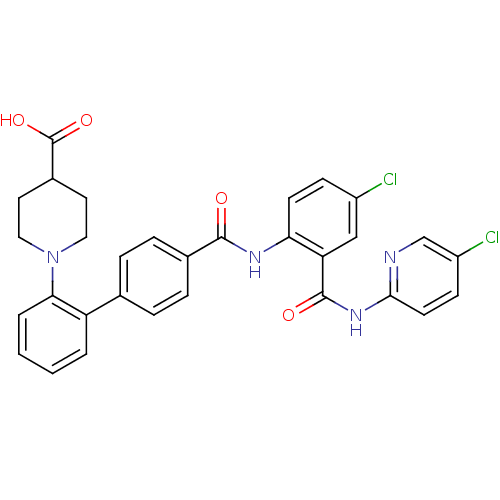
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124984
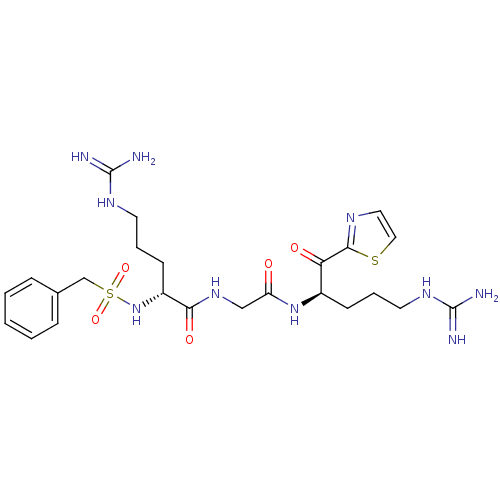
((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)33-19(35)14-32-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,34H,4-5,8-11,14-15H2,(H,32,37)(H,33,35)(H4,25,26,30)(H4,27,28,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477
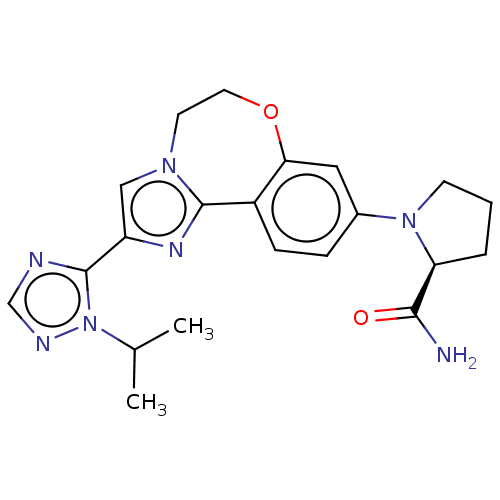
(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(BtCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
| Assay Description
PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... |
bioRxiv (2020)
Article DOI: 10.1101/2020.09.12.293498
BindingDB Entry DOI: 10.7270/Q2VQ352Q |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(BtCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
| Assay Description
PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... |
bioRxiv (2020)
Article DOI: 10.1101/2020.09.12.293498
BindingDB Entry DOI: 10.7270/Q2VQ352Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
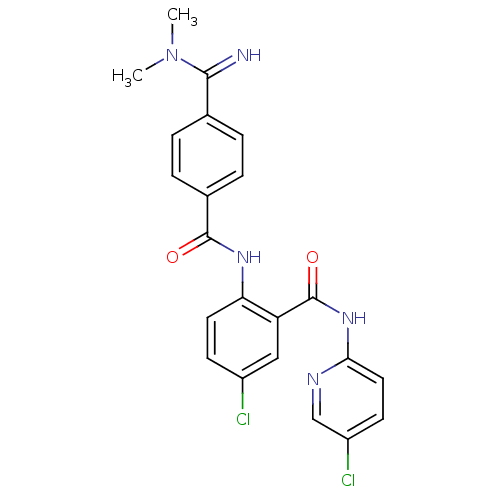
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
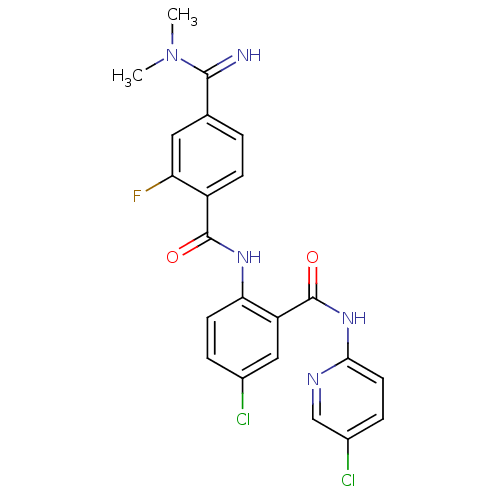
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149482
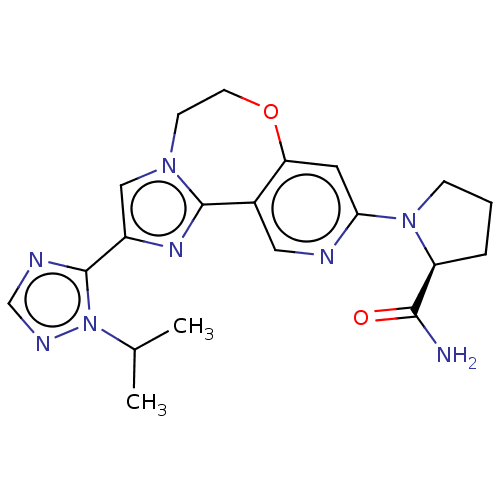
(CHEMBL3770709)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H24N8O2/c1-12(2)28-20(23-11-24-28)14-10-26-6-7-30-16-8-17(22-9-13(16)19(26)25-14)27-5-3-4-15(27)18(21)29/h8-12,15H,3-7H2,1-2H3,(H2,21,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142112
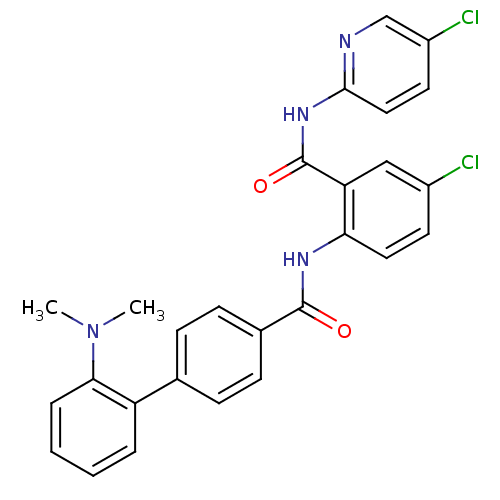
(2'-Dimethylamino-biphenyl-4-carboxylic acid [4-chl...)Show SMILES CN(C)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N4O2/c1-33(2)24-6-4-3-5-21(24)17-7-9-18(10-8-17)26(34)31-23-13-11-19(28)15-22(23)27(35)32-25-14-12-20(29)16-30-25/h3-16H,1-2H3,(H,31,34)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174152
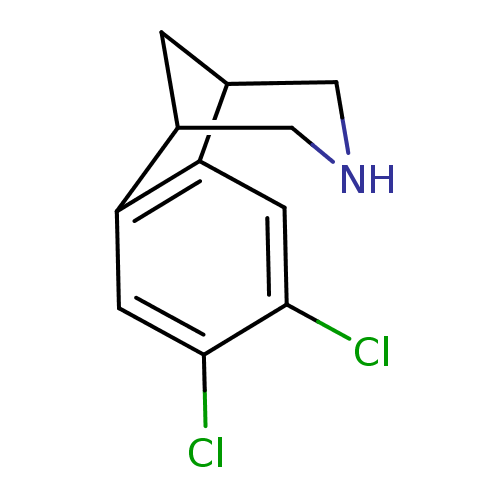
(4,5-Dichloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11Cl2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174139
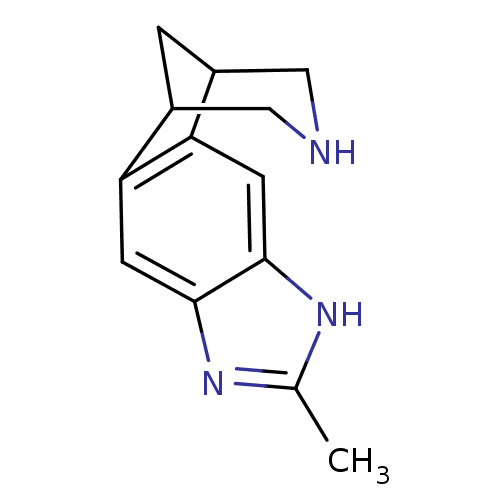
(6-methyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C13H15N3/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149553

(CHEMBL3770306)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H23N7O2/c1-12(2)27-20(22-11-23-27)15-10-25-7-8-29-17-9-13(3-4-14(17)19(25)24-15)26-6-5-16(26)18(21)28/h3-4,9-12,16H,5-8H2,1-2H3,(H2,21,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142139
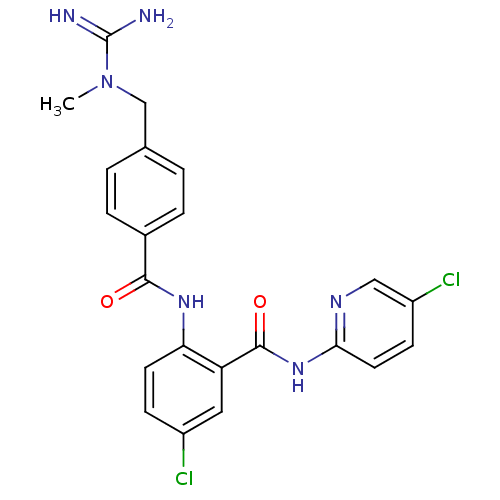
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-{4-[(N-methyl...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(N)=N Show InChI InChI=1S/C22H20Cl2N6O2/c1-30(22(25)26)12-13-2-4-14(5-3-13)20(31)28-18-8-6-15(23)10-17(18)21(32)29-19-9-7-16(24)11-27-19/h2-11H,12H2,1H3,(H3,25,26)(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090
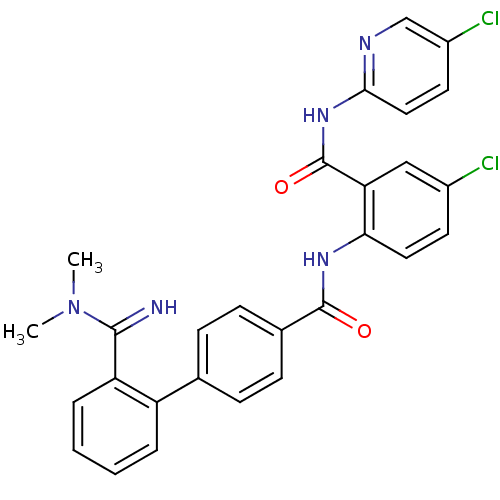
(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142125
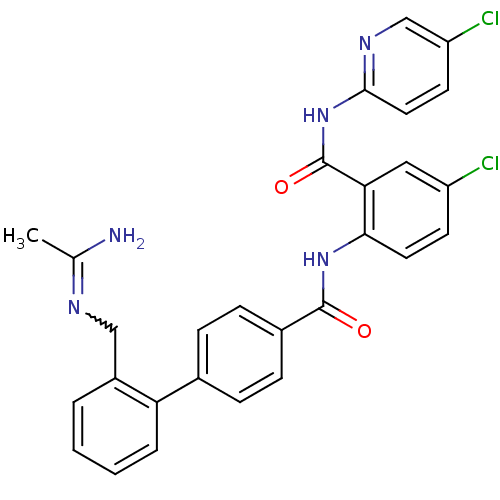
(2'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(N)=NCc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-20-4-2-3-5-23(20)18-6-8-19(9-7-18)27(36)34-25-12-10-21(29)14-24(25)28(37)35-26-13-11-22(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
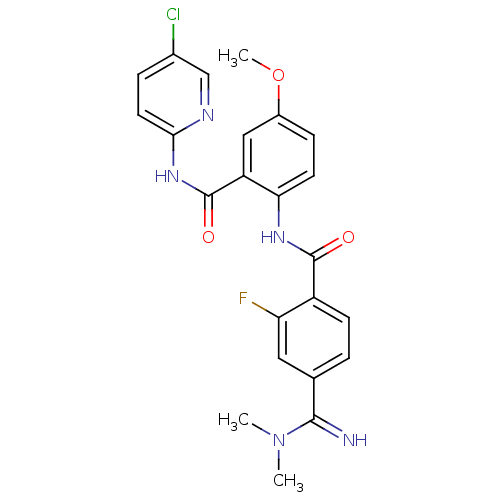
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
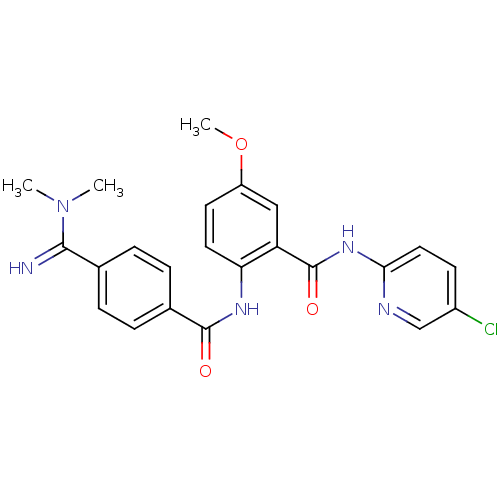
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
| Assay Description
PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... |
bioRxiv (2020)
Article DOI: 10.1101/2020.09.12.293498
BindingDB Entry DOI: 10.7270/Q2VQ352Q |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174145
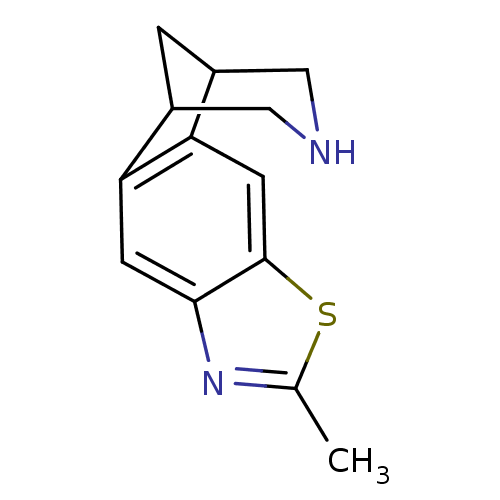
(6-methyl-7-thia-5,13-diazatetracyclo[9.3.1.02,10.0...)Show InChI InChI=1S/C13H14N2S/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174148
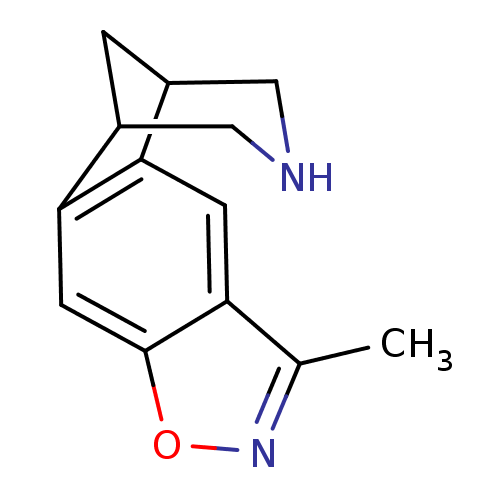
(5-methyl-7-oxa-6,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-10-3-11-8-2-9(6-14-5-8)12(11)4-13(10)16-15-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174154
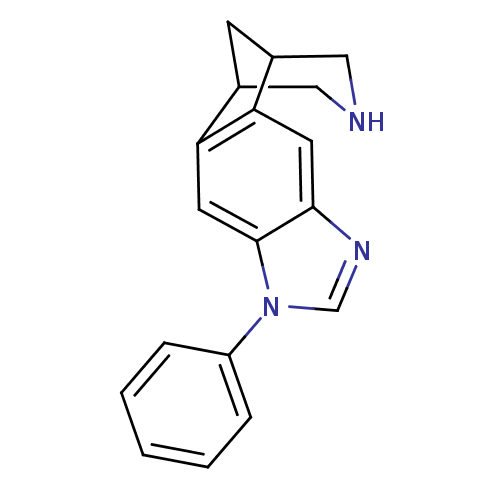
(7-phenyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C18H17N3/c1-2-4-14(5-3-1)21-11-20-17-7-15-12-6-13(10-19-9-12)16(15)8-18(17)21/h1-5,7-8,11-13,19H,6,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174156
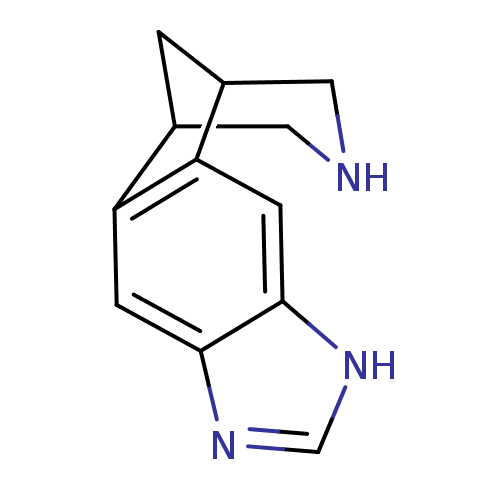
(5,7,13-triazatetracyclo[9.3.1.02,10.04,8]pentadeca...)Show InChI InChI=1S/C12H13N3/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174190
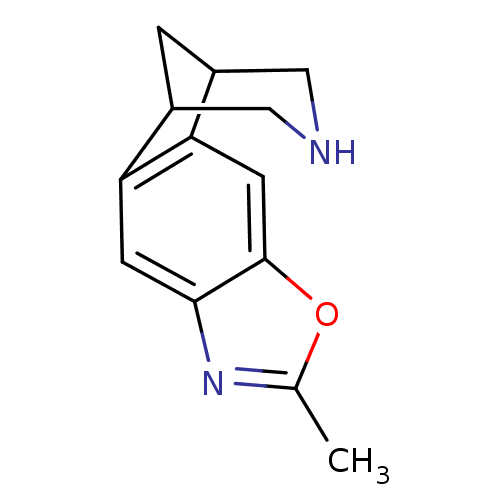
(6-methyl-7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174170
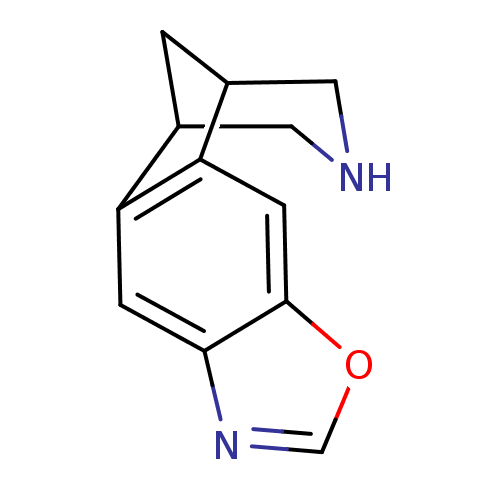
(7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04,8]pentad...)Show InChI InChI=1S/C12H12N2O/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174141
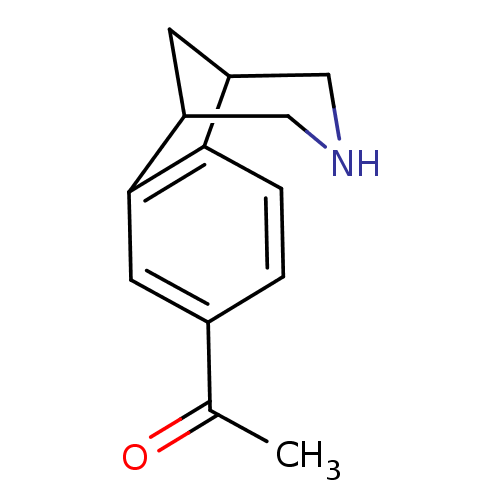
(1-(10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-tr...)Show InChI InChI=1S/C13H15NO/c1-8(15)9-2-3-12-10-4-11(7-14-6-10)13(12)5-9/h2-3,5,10-11,14H,4,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124975
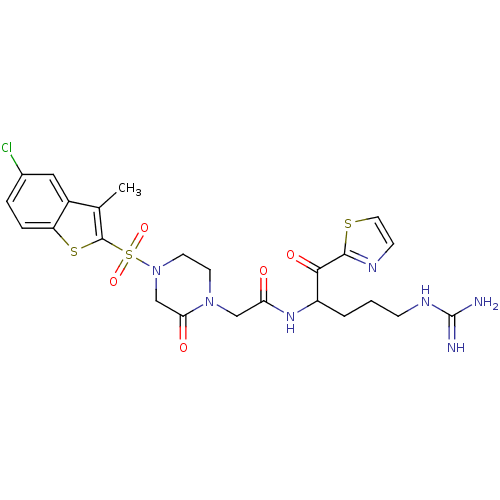
(2-[4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfon...)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)N1CCN(CC(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)C(=O)C1 Show InChI InChI=1S/C24H28ClN7O5S3/c1-14-16-11-15(25)4-5-18(16)39-23(14)40(36,37)32-9-8-31(20(34)13-32)12-19(33)30-17(3-2-6-29-24(26)27)21(35)22-28-7-10-38-22/h4-5,7,10-11,17H,2-3,6,8-9,12-13H2,1H3,(H,30,33)(H4,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113598
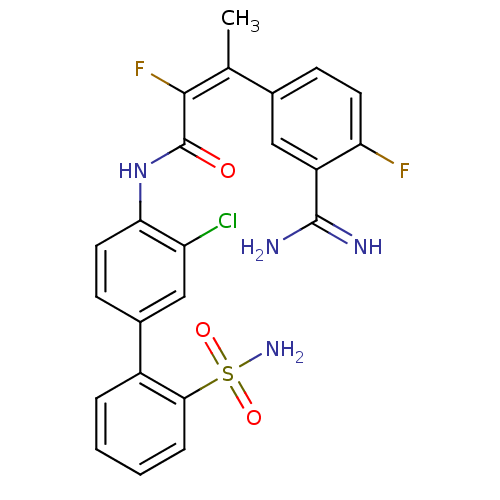
((Z)-3-(3-Carbamimidoyl-4-fluoro-phenyl)-2-fluoro-b...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1ccc(F)c(c1)C(N)=N Show InChI InChI=1S/C23H19ClF2N4O3S/c1-12(13-6-8-18(25)16(10-13)22(27)28)21(26)23(31)30-19-9-7-14(11-17(19)24)15-4-2-3-5-20(15)34(29,32)33/h2-11H,1H3,(H3,27,28)(H,30,31)(H2,29,32,33)/b21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against factor Xa,activity expressed as Ki nM |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174163
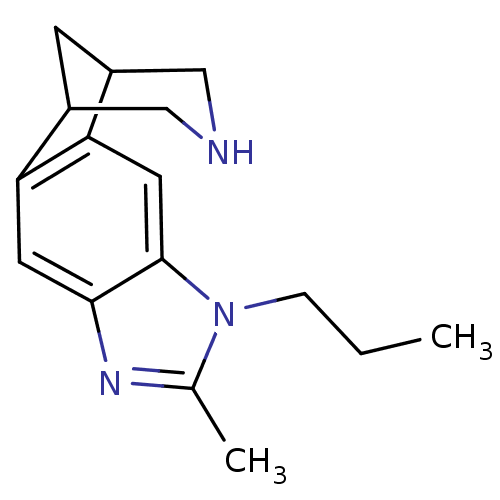
(6-methyl-7-propyl-5,7,13-triazatetracyclo[9.3.1.02...)Show InChI InChI=1S/C16H21N3/c1-3-4-19-10(2)18-15-6-13-11-5-12(9-17-8-11)14(13)7-16(15)19/h6-7,11-12,17H,3-5,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142169
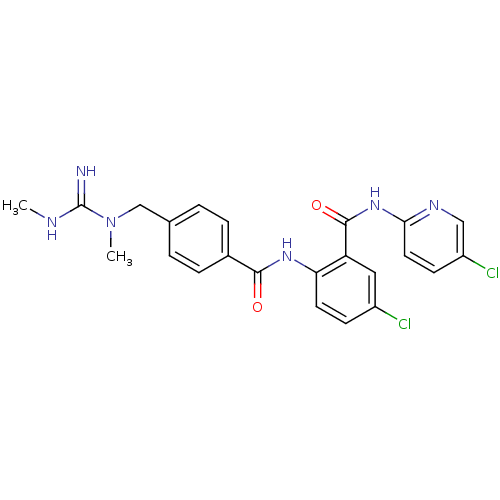
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N'-dime...)Show SMILES CNC(=N)N(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N6O2/c1-27-23(26)31(2)13-14-3-5-15(6-4-14)21(32)29-19-9-7-16(24)11-18(19)22(33)30-20-10-8-17(25)12-28-20/h3-12H,13H2,1-2H3,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142129
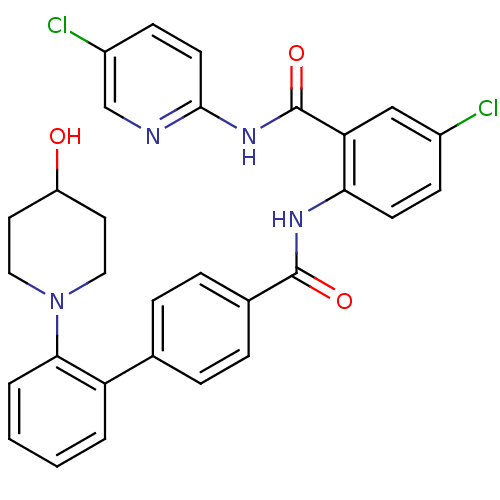
(2'-(4-Hydroxy-piperidin-1-yl)-biphenyl-4-carboxyli...)Show SMILES OC1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H26Cl2N4O3/c31-21-9-11-26(25(17-21)30(39)35-28-12-10-22(32)18-33-28)34-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)36-15-13-23(37)14-16-36/h1-12,17-18,23,37H,13-16H2,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149483
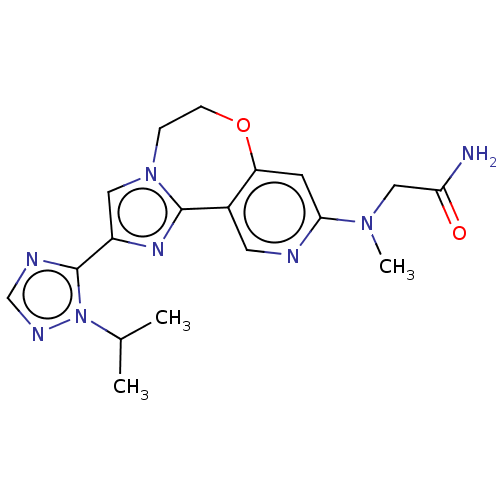
(CHEMBL3770325)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N(C)CC(N)=O Show InChI InChI=1S/C18H22N8O2/c1-11(2)26-18(21-10-22-26)13-8-25-4-5-28-14-6-16(24(3)9-15(19)27)20-7-12(14)17(25)23-13/h6-8,10-11H,4-5,9H2,1-3H3,(H2,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142118
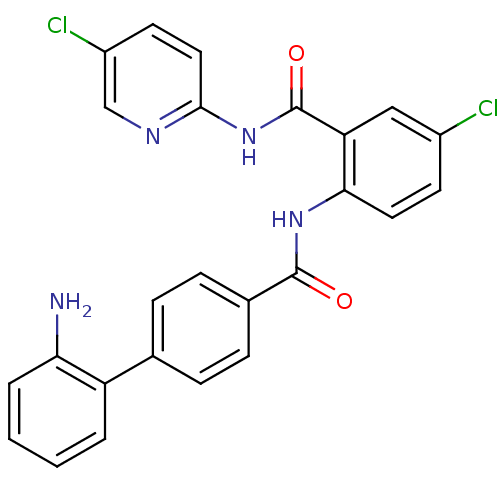
(2'-Amino-biphenyl-4-carboxylic acid [4-chloro-2-(5...)Show SMILES Nc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H18Cl2N4O2/c26-17-9-11-22(20(13-17)25(33)31-23-12-10-18(27)14-29-23)30-24(32)16-7-5-15(6-8-16)19-3-1-2-4-21(19)28/h1-14H,28H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149548
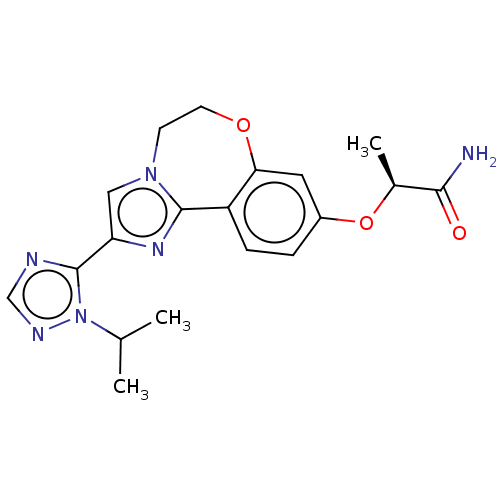
(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174144
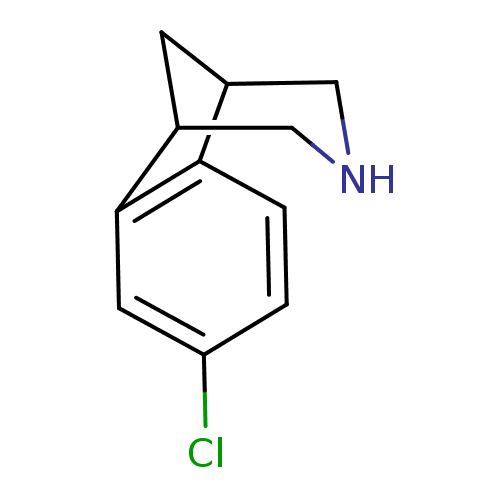
(4-Chloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),...)Show InChI InChI=1S/C11H12ClN/c12-9-1-2-10-7-3-8(6-13-5-7)11(10)4-9/h1-2,4,7-8,13H,3,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174147
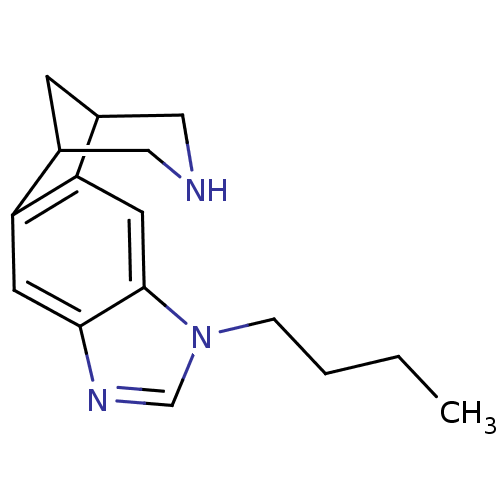
(7-butyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]p...)Show InChI InChI=1S/C16H21N3/c1-2-3-4-19-10-18-15-6-13-11-5-12(9-17-8-11)14(13)7-16(15)19/h6-7,10-12,17H,2-5,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50143282
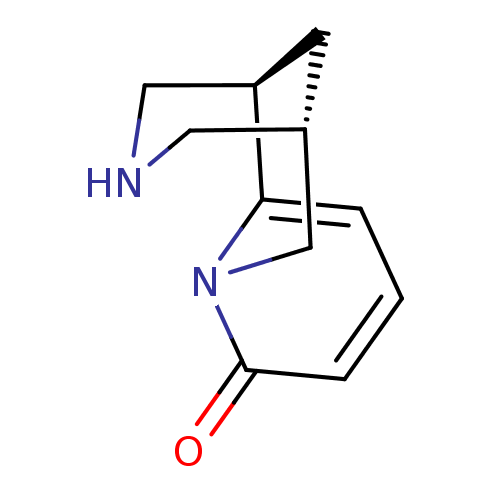
((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2/t8?,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... |
Science 374: 1-13 (2021)
BindingDB Entry DOI: 10.7270/Q23T9MCM |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
| Assay Description
PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... |
bioRxiv (2020)
Article DOI: 10.1101/2020.09.12.293498
BindingDB Entry DOI: 10.7270/Q2VQ352Q |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174158
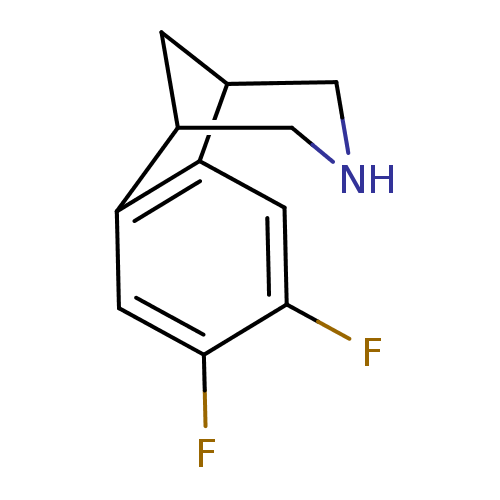
(4,5-Difluoro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11F2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174142
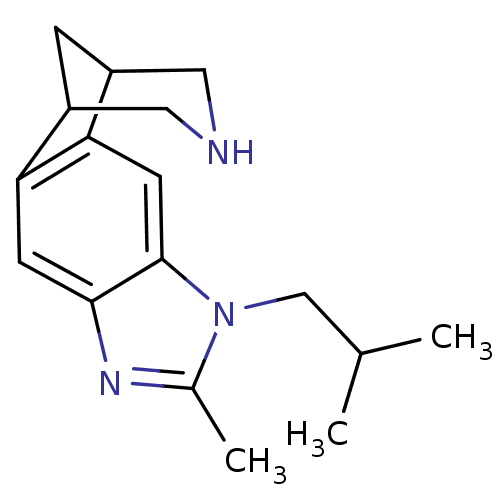
(7-isobutyl-6-methyl-5,7,13-triazatetracyclo[9.3.1....)Show InChI InChI=1S/C17H23N3/c1-10(2)9-20-11(3)19-16-5-14-12-4-13(8-18-7-12)15(14)6-17(16)20/h5-6,10,12-13,18H,4,7-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142142

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N',N'-dim...)Show SMILES CN(C)NCc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H21Cl2N5O2/c1-29(2)26-12-14-3-5-15(6-4-14)21(30)27-19-9-7-16(23)11-18(19)22(31)28-20-10-8-17(24)13-25-20/h3-11,13,26H,12H2,1-2H3,(H,27,30)(H,25,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142161
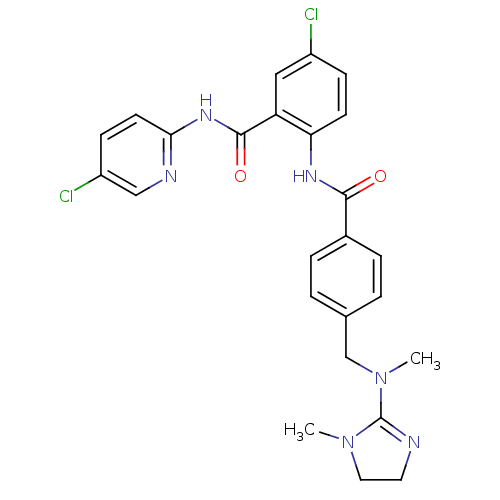
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-{[methyl-(...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C1=NCCN1C |t:32| Show InChI InChI=1S/C25H24Cl2N6O2/c1-32-12-11-28-25(32)33(2)15-16-3-5-17(6-4-16)23(34)30-21-9-7-18(26)13-20(21)24(35)31-22-10-8-19(27)14-29-22/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149476
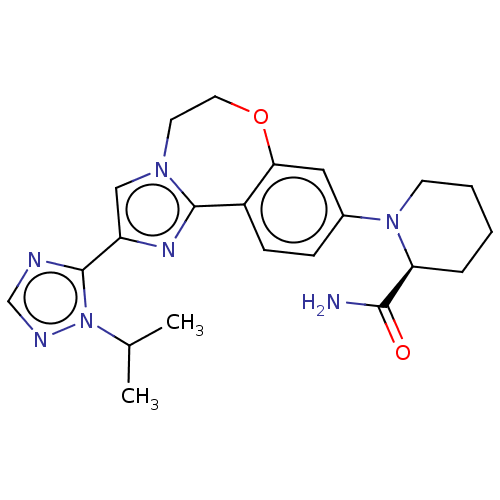
(CHEMBL3770717)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C22H27N7O2/c1-14(2)29-22(24-13-25-29)17-12-27-9-10-31-19-11-15(6-7-16(19)21(27)26-17)28-8-4-3-5-18(28)20(23)30/h6-7,11-14,18H,3-5,8-10H2,1-2H3,(H2,23,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142126
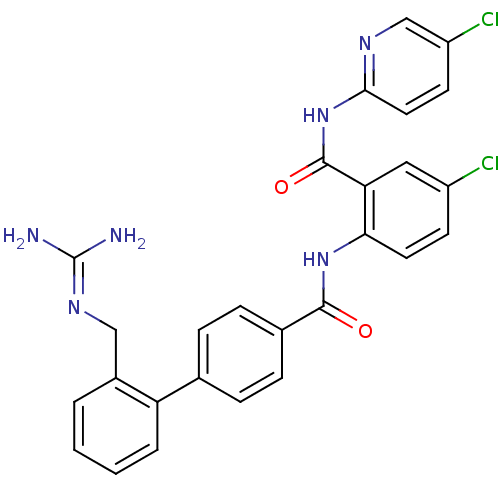
(2'-Guanidinomethyl-biphenyl-4-carboxylic acid [4-c...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1ccccc1-c1ccc(cc1)-[#6](=O)-[#7]-c1ccc(Cl)cc1-[#6](=O)-[#7]-c1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N6O2/c28-19-9-11-23(22(13-19)26(37)35-24-12-10-20(29)15-32-24)34-25(36)17-7-5-16(6-8-17)21-4-2-1-3-18(21)14-33-27(30)31/h1-13,15H,14H2,(H,34,36)(H4,30,31,33)(H,32,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174176
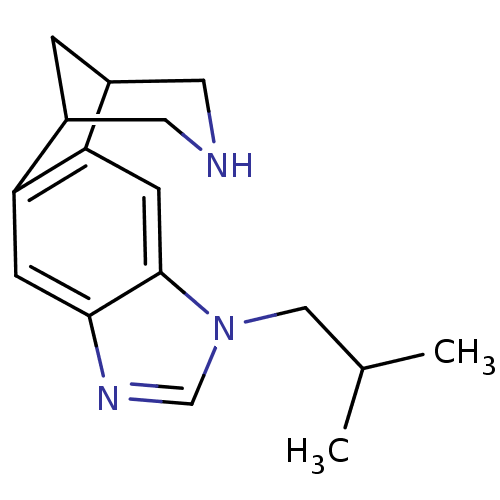
(7-isobutyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,...)Show InChI InChI=1S/C16H21N3/c1-10(2)8-19-9-18-15-4-13-11-3-12(7-17-6-11)14(13)5-16(15)19/h4-5,9-12,17H,3,6-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data