

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528147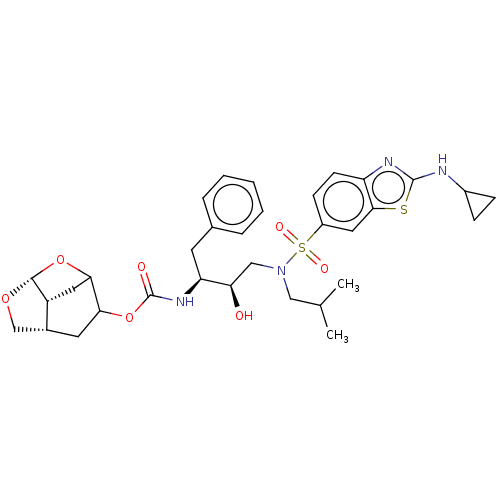 (CHEMBL4514504) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528152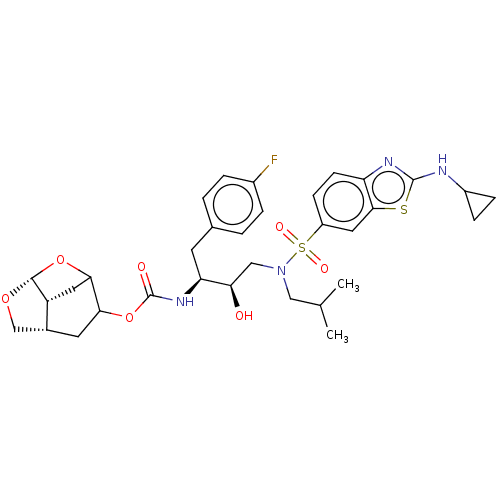 (CHEMBL4532946) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457611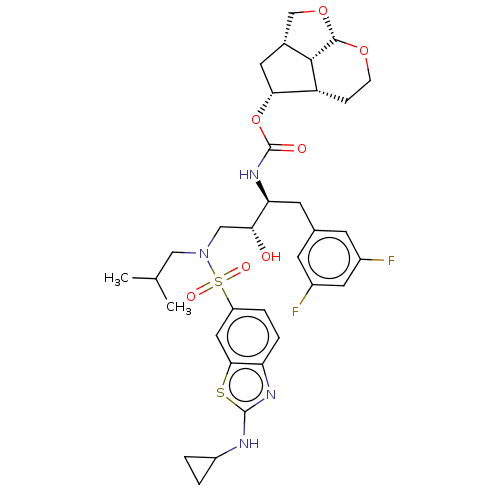 (CHEMBL4214453) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457604 (CHEMBL4213229) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528144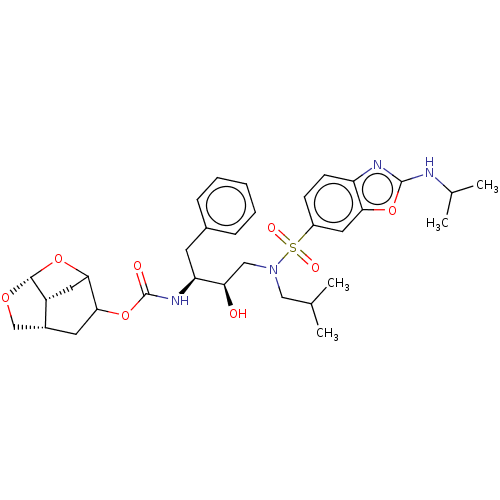 (CHEMBL4435411) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528145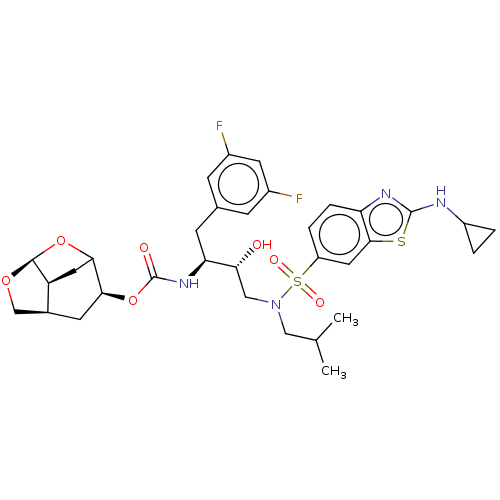 (CHEMBL4586218) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528154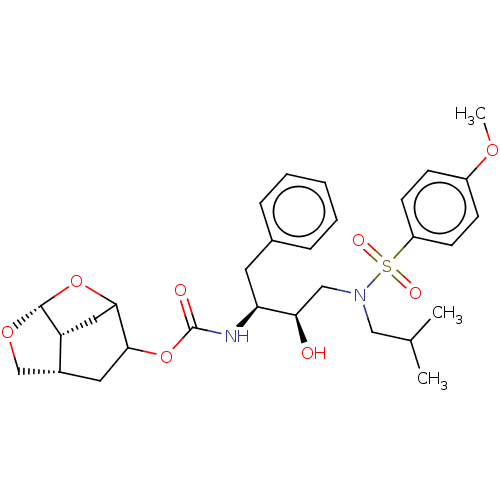 (CHEMBL4545005) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520752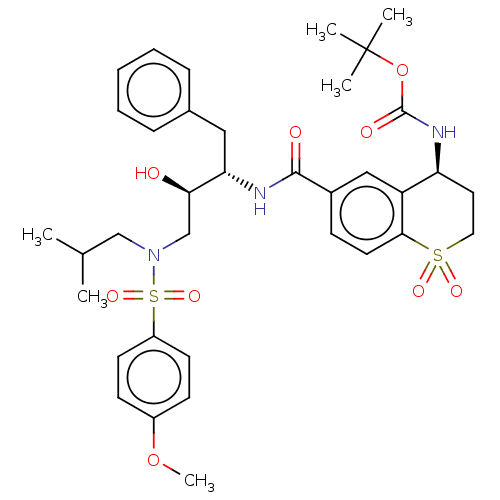 (CHEMBL4474261) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528150 (CHEMBL4449179) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457612 (CHEMBL4218164) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457608 (CHEMBL4211505) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457610 (CHEMBL4207145) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561628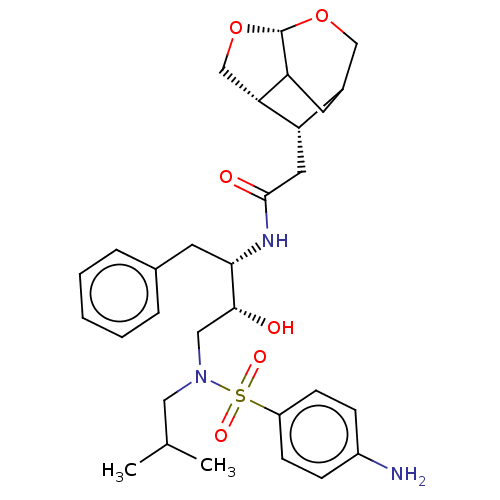 (CHEMBL4800615) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528151 (CHEMBL4533315) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528146 (CHEMBL4437746) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561630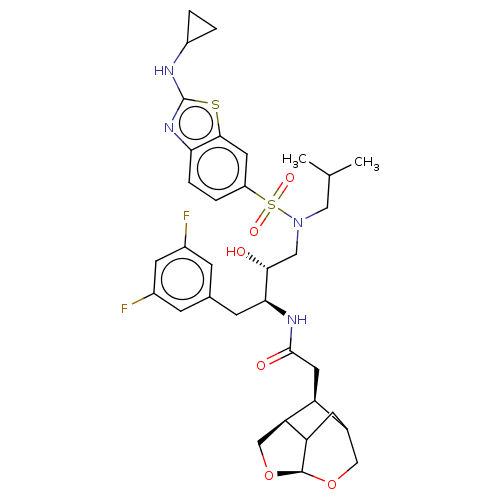 (CHEMBL4743665) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561627 (CHEMBL4744116) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528148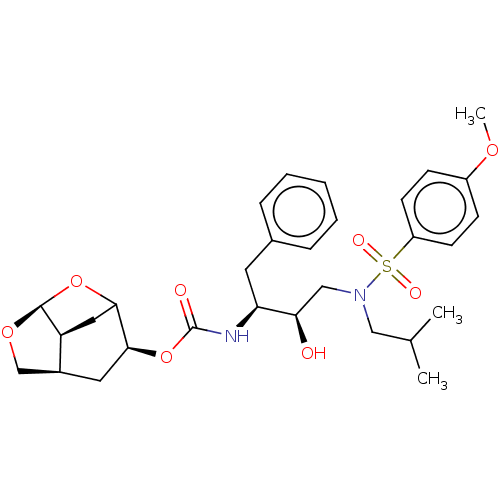 (CHEMBL4469549) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528155 (CHEMBL4460799) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457613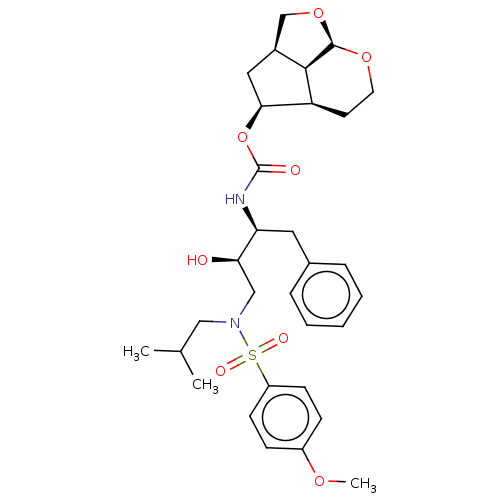 (CHEMBL4205056) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528149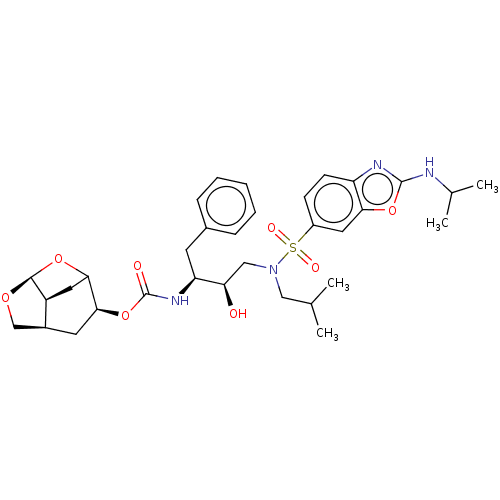 (CHEMBL4568050) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457605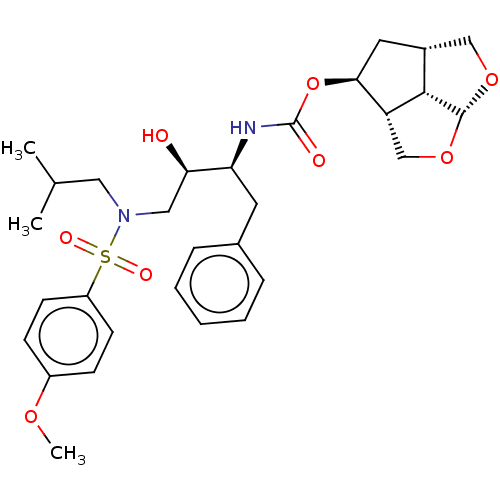 (CHEMBL4210992) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457603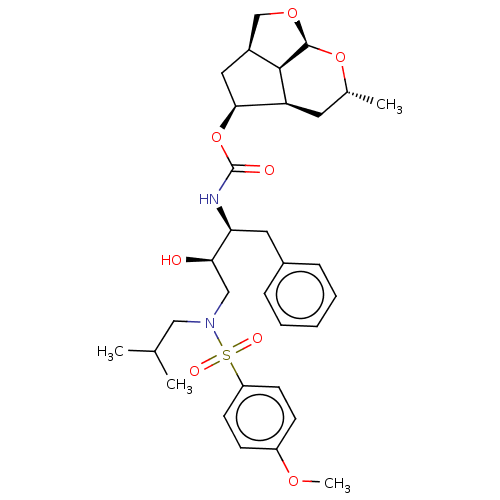 (CHEMBL4217920) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528153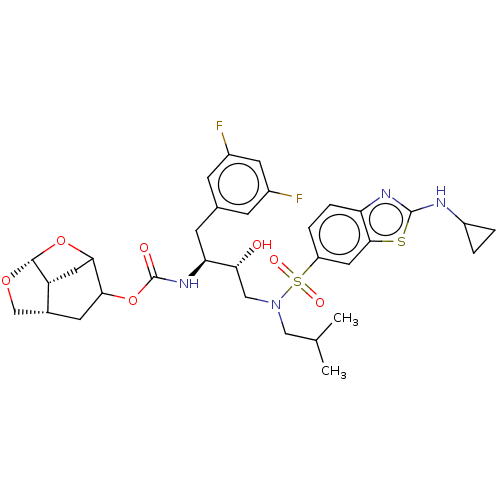 (CHEMBL4592624) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520751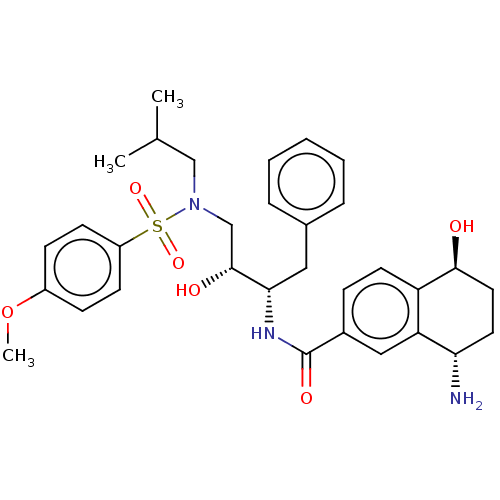 (CHEMBL4473891) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561629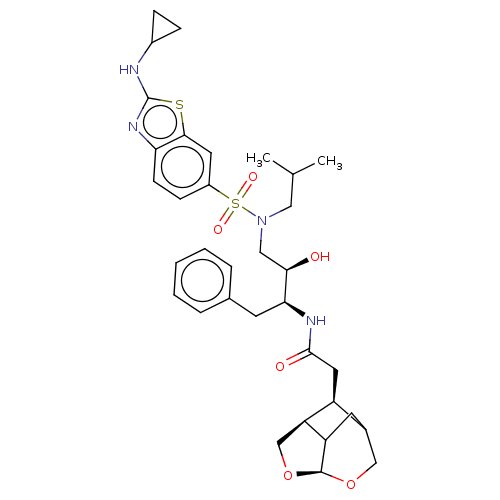 (CHEMBL4740468) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457602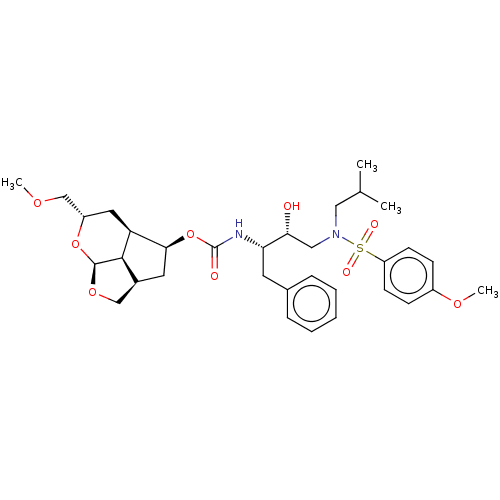 (CHEMBL4214741) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520749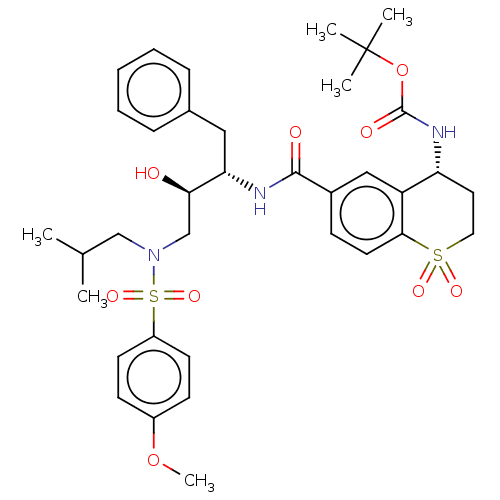 (CHEMBL4459250) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520742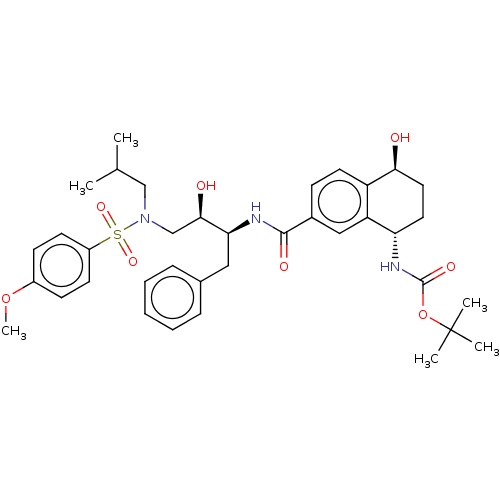 (CHEMBL4526105) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561623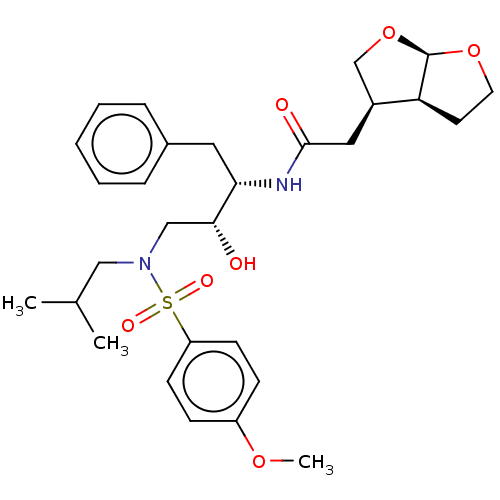 (CHEMBL256107 | GRL-0026A) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520745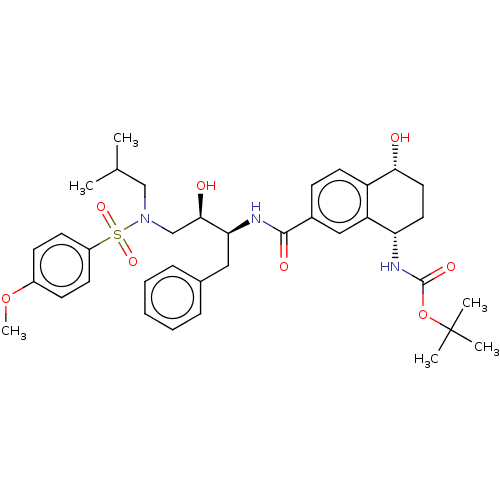 (CHEMBL4444395) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520747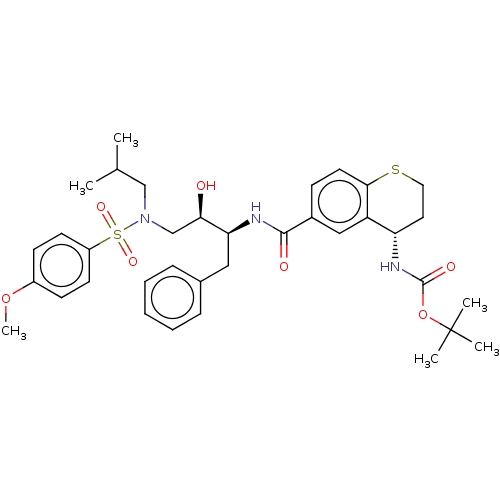 (CHEMBL4532723) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457606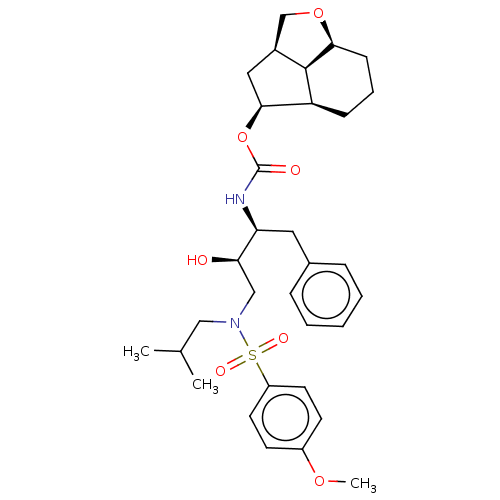 (CHEMBL4215950) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457609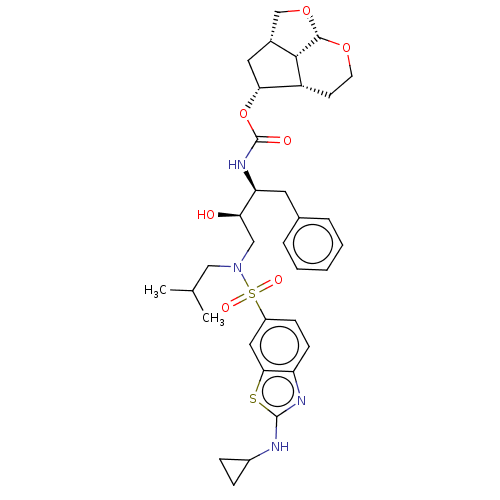 (CHEMBL4218875) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522184 (CHEMBL4444017) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520750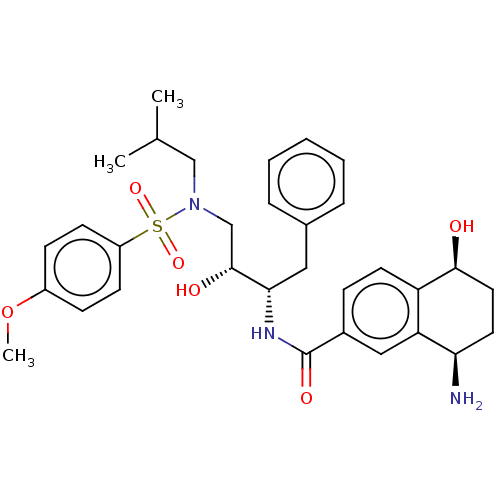 (CHEMBL4452621) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561626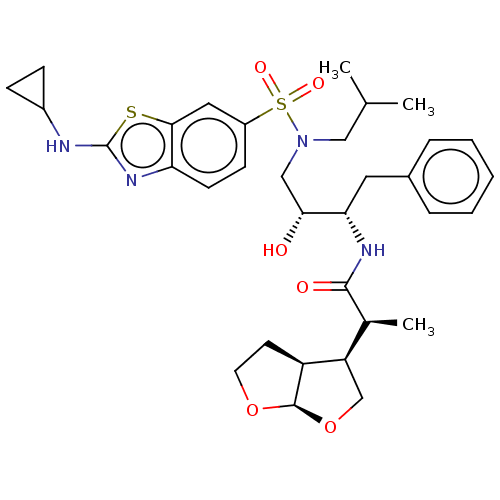 (CHEMBL4763723) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520743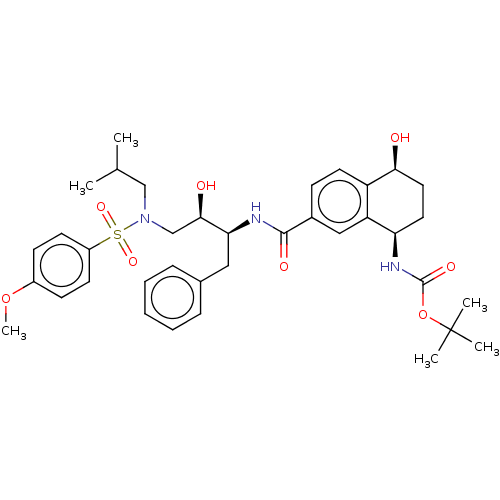 (CHEMBL4444545) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522181 (CHEMBL4456725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457607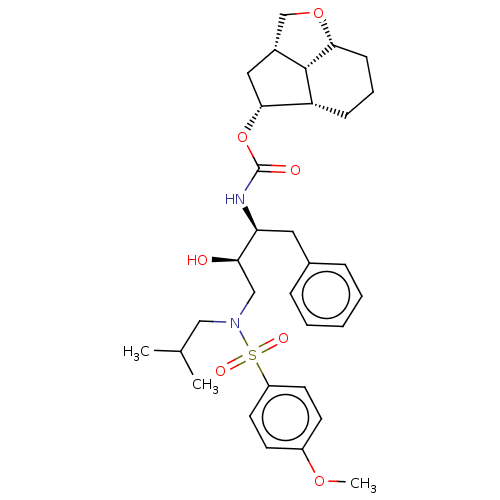 (CHEMBL4206617) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522183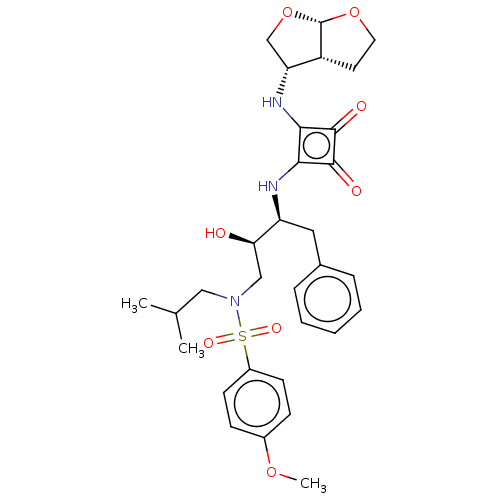 (CHEMBL4467544) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520746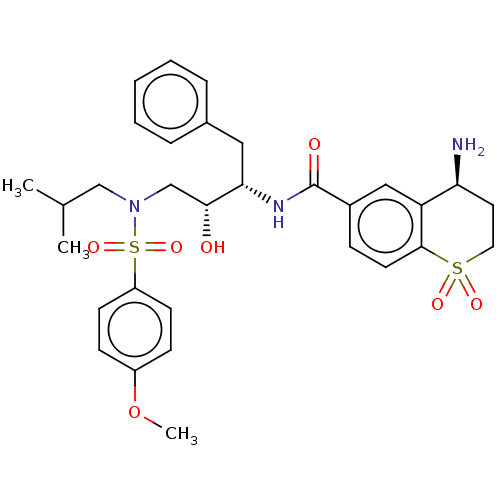 (CHEMBL4569180) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520744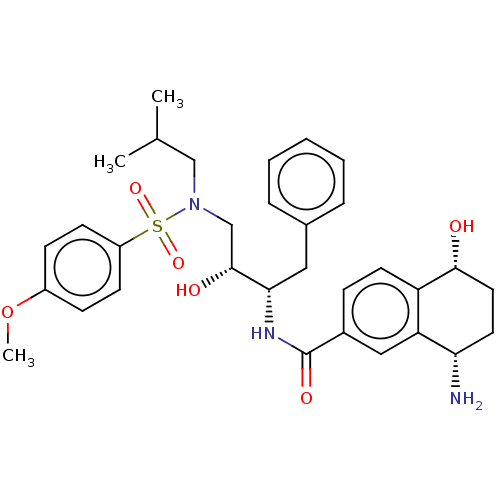 (CHEMBL4444772) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561624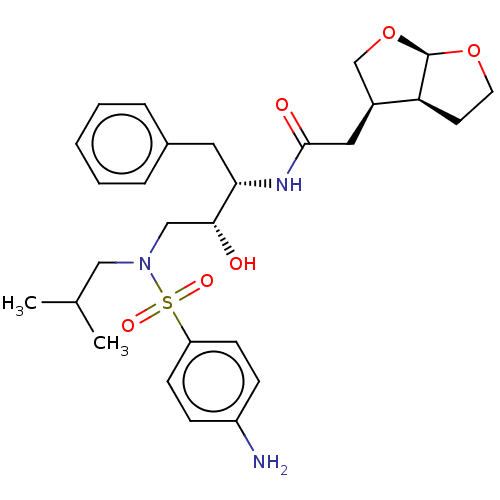 (CHEMBL4764958) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520748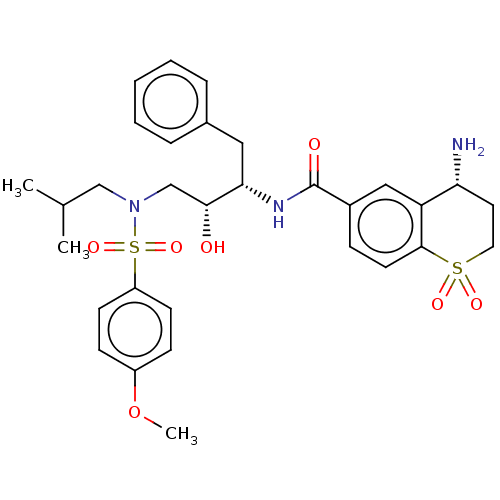 (CHEMBL4588251) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520741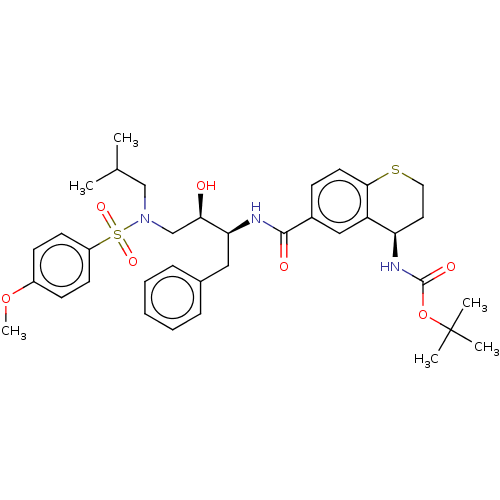 (CHEMBL4561948) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522186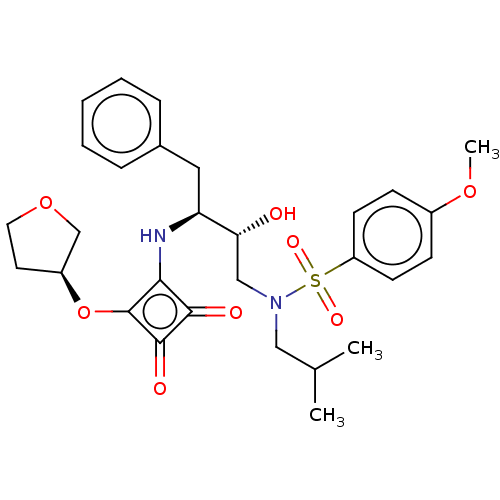 (CHEMBL4445644) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |