 Found 65 hits with Last Name = 'mirzaie' and Initial = 's'
Found 65 hits with Last Name = 'mirzaie' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50422743
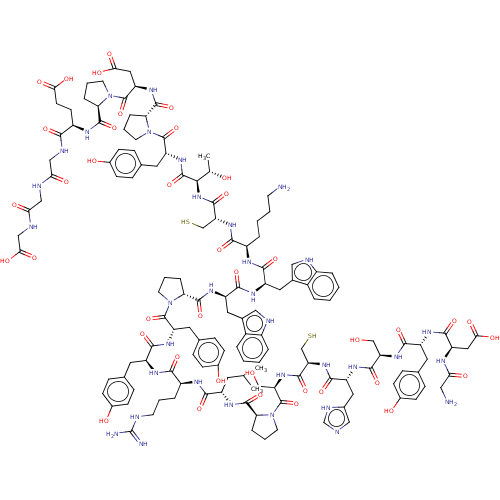
(CHEMBL5275219)Show InChI InChI=1S/C16H22N2OS/c1-2-3-4-5-9-12-18-15(19)14(17-16(18)20)13-10-7-6-8-11-13/h6-8,10-11,19H,2-5,9,12H2,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21008
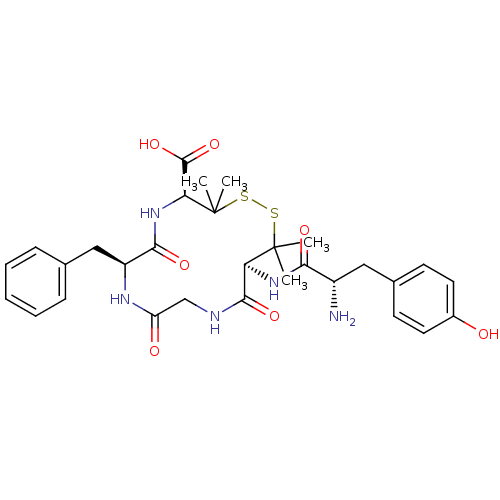
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]lleDelt2 from delta opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50535399

(CHEMBL4473857)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C\C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,t:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7+;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50535398

(CHEMBL4434948)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C/C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,c:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7-;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50231830

(CHEMBL4066390)Show SMILES OC(=O)C(F)(F)F.CC1(C)SCc2ccccc2CSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O |r| Show InChI InChI=1S/C38H47N5O7S2.C2HF3O2/c1-37(2)31(42-33(46)28(39)18-24-14-16-27(44)17-15-24)35(48)40-20-30(45)41-29(19-23-10-6-5-7-11-23)34(47)43-32(36(49)50)38(3,4)52-22-26-13-9-8-12-25(26)21-51-37;3-2(4,5)1(6)7/h5-17,28-29,31-32,44H,18-22,39H2,1-4H3,(H,40,48)(H,41,45)(H,42,46)(H,43,47)(H,49,50);(H,6,7)/t28-,29-,31-,32-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]lleDelt2 from delta opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50231828

(CHEMBL4086190)Show SMILES OC(=O)C(F)(F)F.CC1(C)SCc2cccc(CSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@H]1C(O)=O)c2 |r| Show InChI InChI=1S/C38H47N5O7S2.C2HF3O2/c1-37(2)31(42-33(46)28(39)18-24-13-15-27(44)16-14-24)35(48)40-20-30(45)41-29(19-23-9-6-5-7-10-23)34(47)43-32(36(49)50)38(3,4)52-22-26-12-8-11-25(17-26)21-51-37;3-2(4,5)1(6)7/h5-17,28-29,31-32,44H,18-22,39H2,1-4H3,(H,40,48)(H,41,45)(H,42,46)(H,43,47)(H,49,50);(H,6,7)/t28-,29-,31-,32-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]lleDelt2 from delta opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50535399

(CHEMBL4473857)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C\C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,t:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7+;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50535398

(CHEMBL4434948)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C/C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,c:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7-;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50231830

(CHEMBL4066390)Show SMILES OC(=O)C(F)(F)F.CC1(C)SCc2ccccc2CSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O |r| Show InChI InChI=1S/C38H47N5O7S2.C2HF3O2/c1-37(2)31(42-33(46)28(39)18-24-14-16-27(44)17-15-24)35(48)40-20-30(45)41-29(19-23-10-6-5-7-11-23)34(47)43-32(36(49)50)38(3,4)52-22-26-13-9-8-12-25(26)21-51-37;3-2(4,5)1(6)7/h5-17,28-29,31-32,44H,18-22,39H2,1-4H3,(H,40,48)(H,41,45)(H,42,46)(H,43,47)(H,49,50);(H,6,7)/t28-,29-,31-,32-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50231829
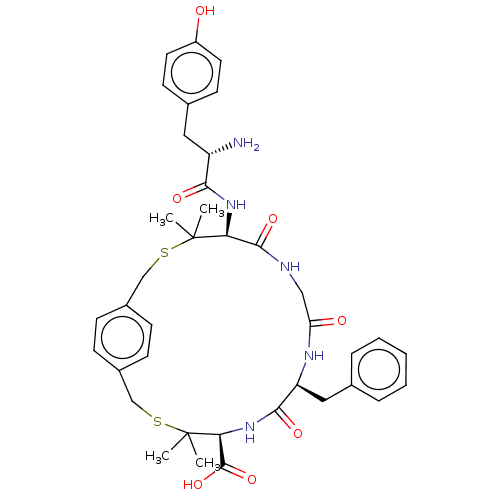
(CHEMBL4104096)Show SMILES OC(=O)C(F)(F)F.CC1(C)SCc2ccc(CSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@H]1C(O)=O)cc2 |r| Show InChI InChI=1S/C38H47N5O7S2.C2HF3O2/c1-37(2)31(42-33(46)28(39)18-24-14-16-27(44)17-15-24)35(48)40-20-30(45)41-29(19-23-8-6-5-7-9-23)34(47)43-32(36(49)50)38(3,4)52-22-26-12-10-25(11-13-26)21-51-37;3-2(4,5)1(6)7/h5-17,28-29,31-32,44H,18-22,39H2,1-4H3,(H,40,48)(H,41,45)(H,42,46)(H,43,47)(H,49,50);(H,6,7)/t28-,29-,31-,32-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50231828

(CHEMBL4086190)Show SMILES OC(=O)C(F)(F)F.CC1(C)SCc2cccc(CSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@H]1C(O)=O)c2 |r| Show InChI InChI=1S/C38H47N5O7S2.C2HF3O2/c1-37(2)31(42-33(46)28(39)18-24-13-15-27(44)16-14-24)35(48)40-20-30(45)41-29(19-23-9-6-5-7-10-23)34(47)43-32(36(49)50)38(3,4)52-22-26-12-8-11-25(17-26)21-51-37;3-2(4,5)1(6)7/h5-17,28-29,31-32,44H,18-22,39H2,1-4H3,(H,40,48)(H,41,45)(H,42,46)(H,43,47)(H,49,50);(H,6,7)/t28-,29-,31-,32-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 292 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21008

((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50535398

(CHEMBL4434948)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C/C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,c:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7-;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 495 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50231829

(CHEMBL4104096)Show SMILES OC(=O)C(F)(F)F.CC1(C)SCc2ccc(CSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@H]1C(O)=O)cc2 |r| Show InChI InChI=1S/C38H47N5O7S2.C2HF3O2/c1-37(2)31(42-33(46)28(39)18-24-14-16-27(44)17-15-24)35(48)40-20-30(45)41-29(19-23-8-6-5-7-9-23)34(47)43-32(36(49)50)38(3,4)52-22-26-12-10-25(11-13-26)21-51-37;3-2(4,5)1(6)7/h5-17,28-29,31-32,44H,18-22,39H2,1-4H3,(H,40,48)(H,41,45)(H,42,46)(H,43,47)(H,49,50);(H,6,7)/t28-,29-,31-,32-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 546 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]lleDelt2 from delta opioid receptor in Wistar rat brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 449-454 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00044
BindingDB Entry DOI: 10.7270/Q22F7QP1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50535399

(CHEMBL4473857)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C\C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,t:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7+;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 686 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50233798
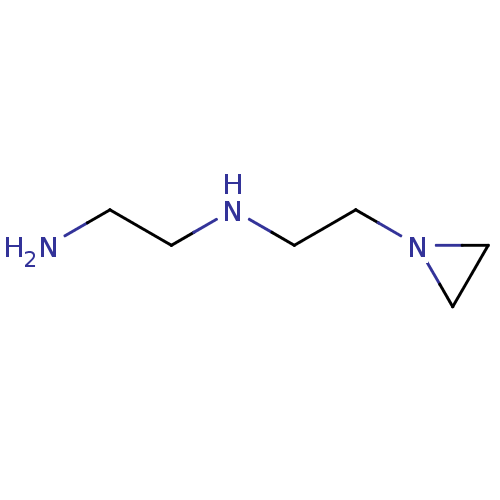
(CHEMBL398940 | N-(2-aminoethyl)-1-aziridine-ethana...)Show InChI InChI=1S/C6H15N3/c7-1-2-8-3-4-9-5-6-9/h8H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 4.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50552672
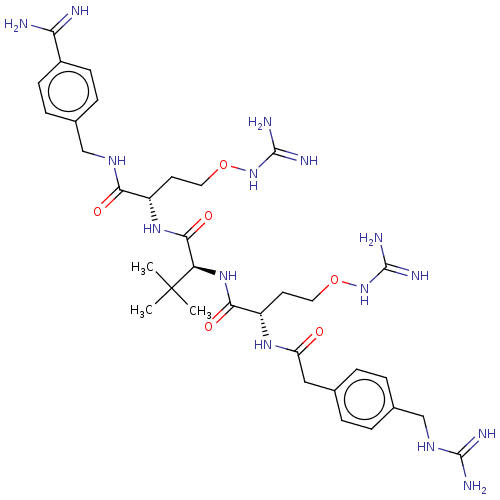
(CHEMBL4790628)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCONC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCONC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50535399

(CHEMBL4473857)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C\C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,t:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7+;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cell membranes assessed as reduction in SNC80-induced [35S]GTPgammaS binding incu... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50370067
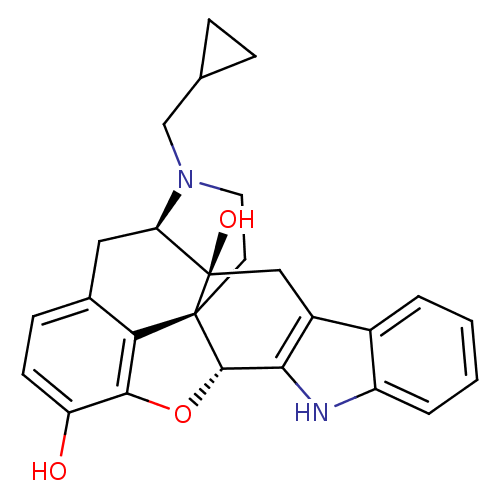
(CHEMBL1237164)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2ccccc2c1C[C@@]35O |r,TLB:28:29:7.12.13:4.5.18,30:29:7.12.13:4.5.18| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as reduction in SNC80-induced inhibition of forskolin stimulated c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50229129
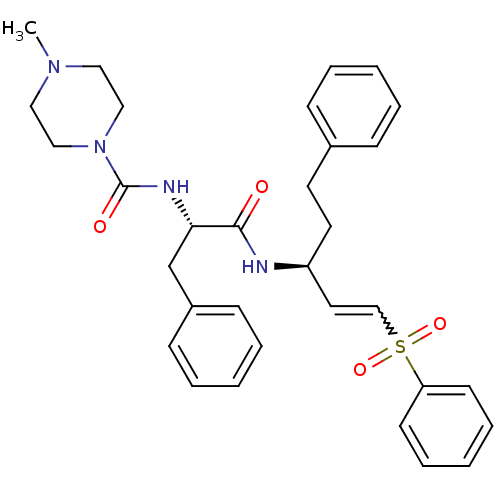
(4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:31.34| Show InChI InChI=1S/C32H38N4O4S/c1-35-20-22-36(23-21-35)32(38)34-30(25-27-13-7-3-8-14-27)31(37)33-28(18-17-26-11-5-2-6-12-26)19-24-41(39,40)29-15-9-4-10-16-29/h2-16,19,24,28,30H,17-18,20-23,25H2,1H3,(H,33,37)(H,34,38)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50421866

(CHEMBL5281591)Show InChI InChI=1S/C17H19N5O/c1-9-5-14(23-3)21-15-10(2)4-11(7-13(9)15)6-12-8-20-17(19)22-16(12)18/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50421871
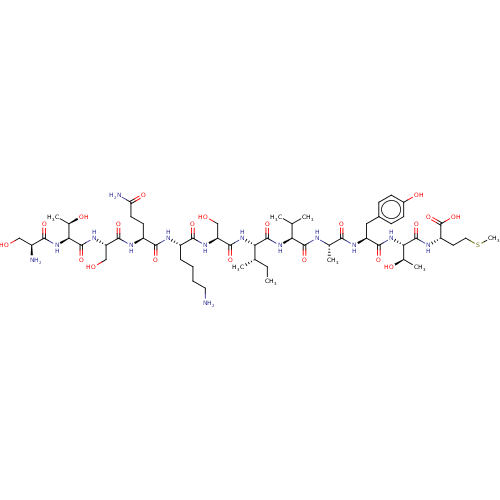
(CHEMBL5273921)Show InChI InChI=1S/C16H17N5O/c1-9-5-14(22-2)20-13-7-10(3-4-12(9)13)6-11-8-19-16(18)21-15(11)17/h3-5,7-8H,6H2,1-2H3,(H4,17,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50073850
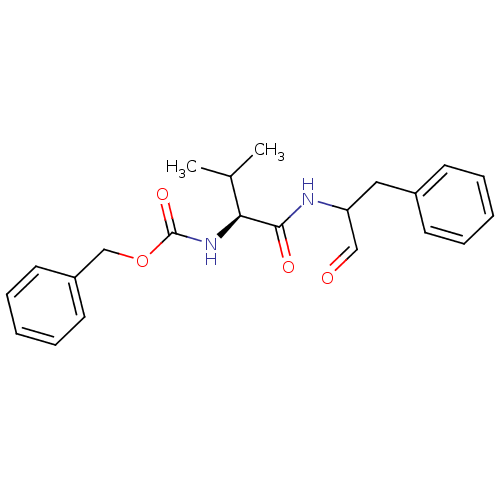
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50421869
(CHEMBL5277148)Show InChI InChI=1S/C17H20N6/c1-10-6-15(23(2)3)21-14-5-4-11(8-13(10)14)7-12-9-20-17(19)22-16(12)18/h4-6,8-9H,7H2,1-3H3,(H4,18,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM51395
(CHEMBL263634 | N-[(2S)-1-[[[2-(3,4-dihydro-2H-quin...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NNC(=O)OCC(=O)N1CCCc2ccccc12 Show InChI InChI=1S/C28H33N5O6/c1-28(2,3)39-26(36)30-22(15-19-16-29-21-12-6-5-11-20(19)21)25(35)31-32-27(37)38-17-24(34)33-14-8-10-18-9-4-7-13-23(18)33/h4-7,9,11-13,16,22,29H,8,10,14-15,17H2,1-3H3,(H,30,36)(H,31,35)(H,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50421870
(CHEMBL5281306)Show InChI InChI=1S/C19H22N6O/c1-12-8-17(25-4-6-26-7-5-25)23-16-10-13(2-3-15(12)16)9-14-11-22-19(21)24-18(14)20/h2-3,8,10-11H,4-7,9H2,1H3,(H4,20,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50422743

(CHEMBL5275219)Show InChI InChI=1S/C16H22N2OS/c1-2-3-4-5-9-12-18-15(19)14(17-16(18)20)13-10-7-6-8-11-13/h6-8,10-11,19H,2-5,9,12H2,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50535398

(CHEMBL4434948)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C/C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,c:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7-;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cell membranes assessed as reduction in SNC80-induced [35S]GTPgammaS binding incu... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50374666
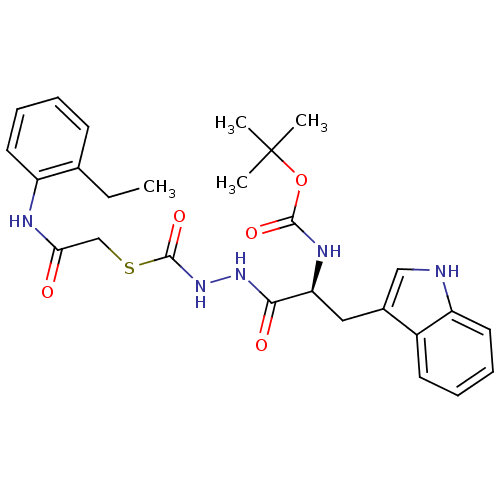
(CHEMBL259450)Show SMILES CCc1ccccc1NC(=O)CSC(=O)NNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C27H33N5O5S/c1-5-17-10-6-8-12-20(17)29-23(33)16-38-26(36)32-31-24(34)22(30-25(35)37-27(2,3)4)14-18-15-28-21-13-9-7-11-19(18)21/h6-13,15,22,28H,5,14,16H2,1-4H3,(H,29,33)(H,30,35)(H,31,34)(H,32,36)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429226
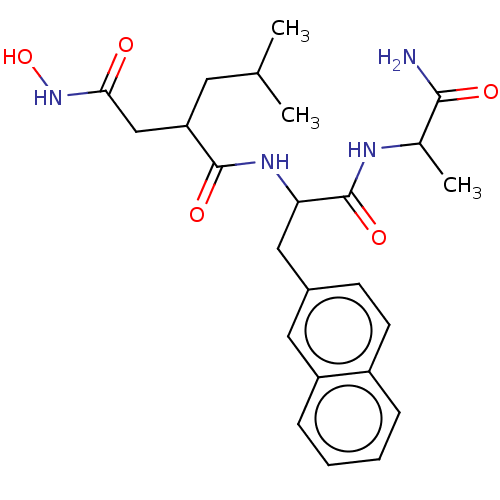
(med.21724, Compound 11)Show SMILES CC(C)CC(CC(=O)NO)C(=O)NC(Cc1ccc2ccccc2c1)C(=O)NC(C)C(N)=O Show InChI InChI=1S/C24H32N4O5/c1-14(2)10-19(13-21(29)28-33)23(31)27-20(24(32)26-15(3)22(25)30)12-16-8-9-17-6-4-5-7-18(17)11-16/h4-9,11,14-15,19-20,33H,10,12-13H2,1-3H3,(H2,25,30)(H,26,32)(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50422742
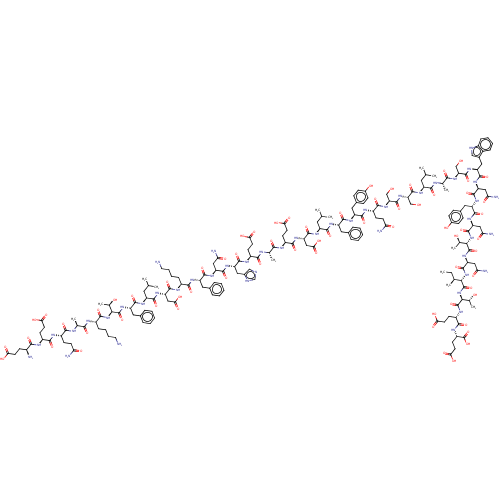
(CHEMBL5274408)Show InChI InChI=1S/C22H18N2O2/c25-20-22(18-12-6-2-7-13-18,19-14-8-3-9-15-19)23-21(26)24(20)16-17-10-4-1-5-11-17/h1-15H,16H2,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cell membranes assessed as reduction in SNC80-induced [35S]GTPgammaS binding incu... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429240
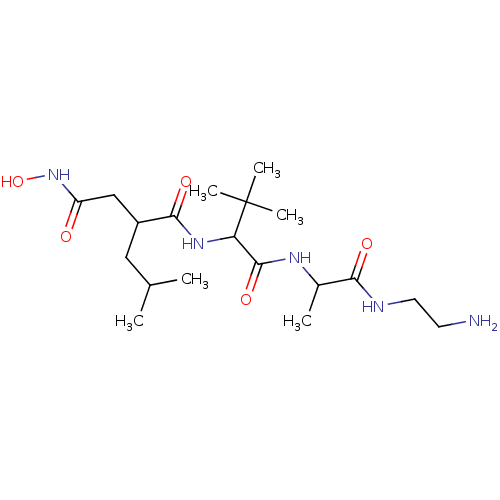
(med.21724, Compound 12)Show SMILES CC(C)CC(CC(=O)NO)C(=O)NC(C(=O)NC(C)C(=O)NCCN)C(C)(C)C Show InChI InChI=1S/C19H37N5O5/c1-11(2)9-13(10-14(25)24-29)17(27)23-15(19(4,5)6)18(28)22-12(3)16(26)21-8-7-20/h11-13,15,29H,7-10,20H2,1-6H3,(H,21,26)(H,22,28)(H,23,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429241
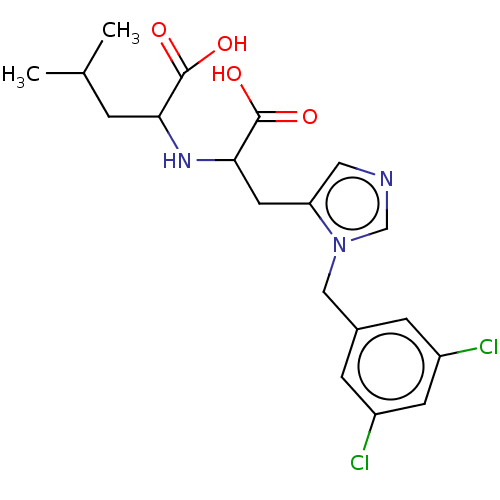
(med.21724, Compound 13)Show SMILES CC(C)CC(NC(Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50421867
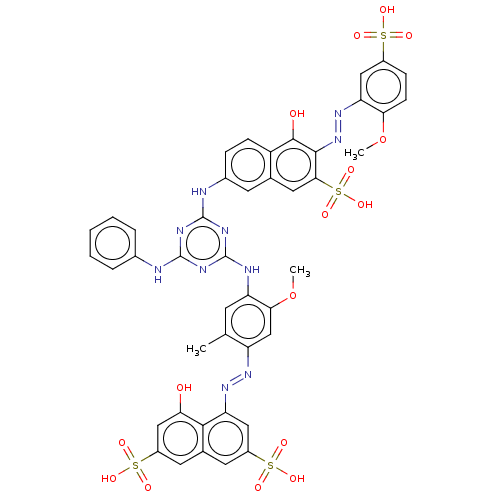
(CHEMBL216826)Show InChI InChI=1S/C18H22N6O/c1-10-5-14(24(2)3)13-7-11(8-15(25-4)16(13)22-10)6-12-9-21-18(20)23-17(12)19/h5,7-9H,6H2,1-4H3,(H4,19,20,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50421865
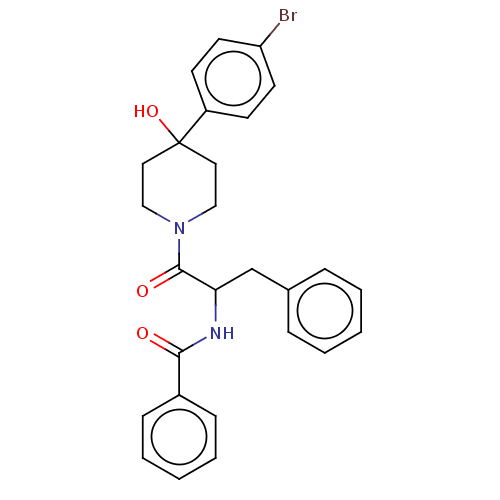
(CHEMBL5270175)Show InChI InChI=1S/C17H19N5O2/c1-9-4-13(23-2)12-6-10(7-14(24-3)15(12)21-9)5-11-8-20-17(19)22-16(11)18/h4,6-8H,5H2,1-3H3,(H4,18,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50467780
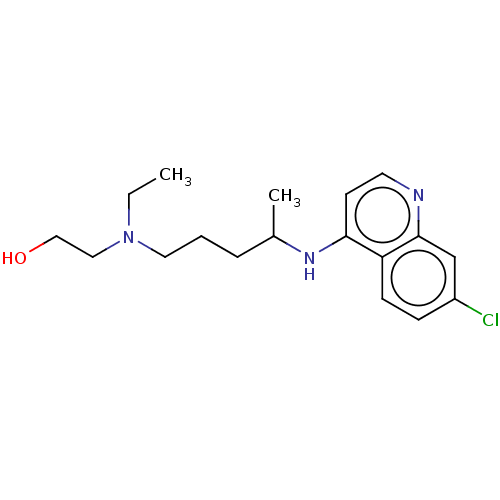
(CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...)Show InChI InChI=1S/C18H26ClN3O/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM22985
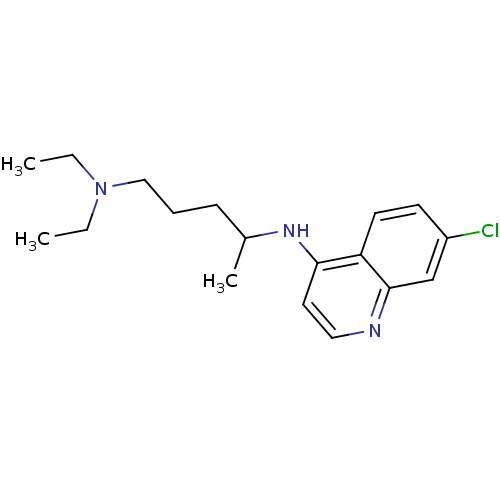
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429290
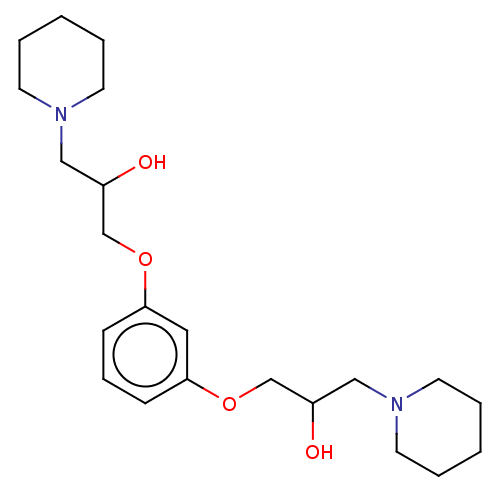
(med.21724, Compound 26)Show InChI InChI=1S/C22H36N2O4/c25-19(15-23-10-3-1-4-11-23)17-27-21-8-7-9-22(14-21)28-18-20(26)16-24-12-5-2-6-13-24/h7-9,14,19-20,25-26H,1-6,10-13,15-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM428874

(med.21724, Compound 1)Show InChI InChI=1S/C16H20N4O2/c1-19-8-10-20(11-9-19)14-4-2-13(3-5-14)12-17-16(21)15-6-7-18-22-15/h2-7H,8-12H2,1H3,(H,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM83797
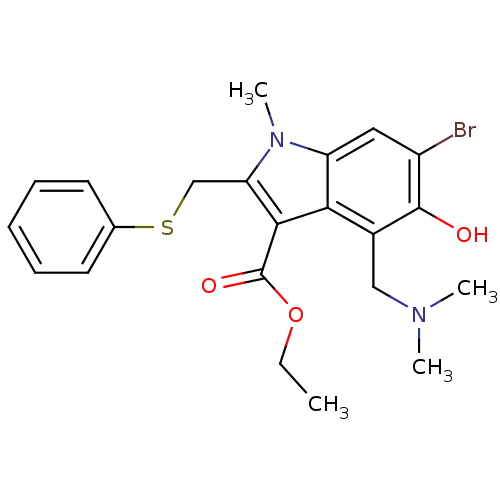
(6-Bromo-4-dimethylaminomethyl-5-hydroxy-1-methyl-2...)Show SMILES CCOC(=O)c1c(CSc2ccccc2)n(C)c2cc(Br)c(O)c(CN(C)C)c12 Show InChI InChI=1S/C22H25BrN2O3S/c1-5-28-22(27)20-18(13-29-14-9-7-6-8-10-14)25(4)17-11-16(23)21(26)15(19(17)20)12-24(2)3/h6-11,26H,5,12-13H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM428875

(med.21724, Compound 2)Show SMILES O[C@@H]1[C@@H](COC(=O)c2cc(O)c(O)c(O)c2)O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C34H28O22/c35-14-1-10(2-15(36)23(14)43)30(48)52-9-22-27(47)28(54-31(49)11-3-16(37)24(44)17(38)4-11)29(55-32(50)12-5-18(39)25(45)19(40)6-12)34(53-22)56-33(51)13-7-20(41)26(46)21(42)8-13/h1-8,22,27-29,34-47H,9H2/t22-,27-,28-,29-,34+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50376204
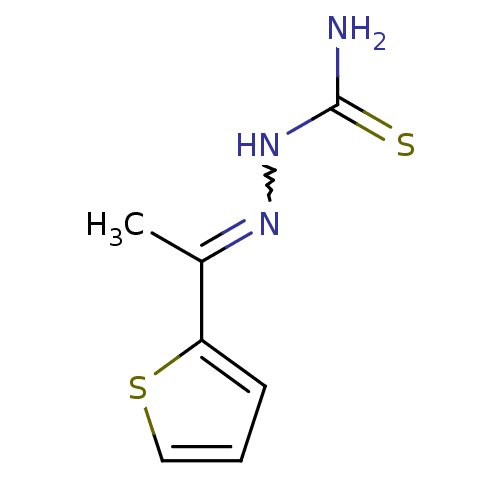
(CHEMBL401925 | med.21724, Compound 23)Show InChI InChI=1S/C7H9N3S2/c1-5(9-10-7(8)11)6-3-2-4-12-6/h2-4H,1H3,(H3,8,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50422742

(CHEMBL5274408)Show InChI InChI=1S/C22H18N2O2/c25-20-22(18-12-6-2-7-13-18,19-14-8-3-9-15-19)23-21(26)24(20)16-17-10-4-1-5-11-17/h1-15H,16H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429289
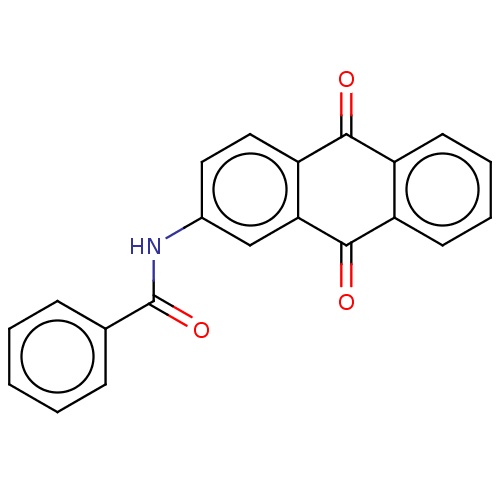
(med.21724, Compound 25)Show InChI InChI=1S/C21H13NO3/c23-19-15-8-4-5-9-16(15)20(24)18-12-14(10-11-17(18)19)22-21(25)13-6-2-1-3-7-13/h1-12H,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50424712
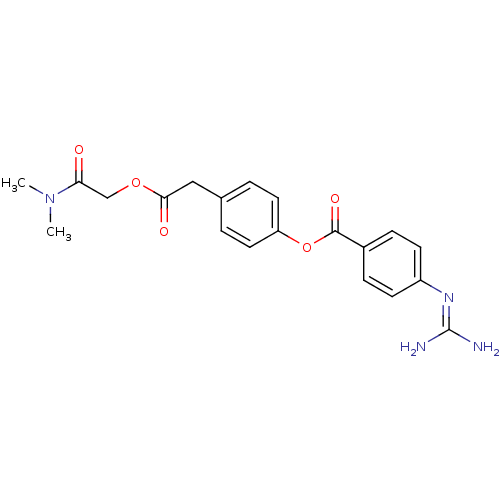
(CAMOSTAT | Camostat Mesilate | Foipan)Show SMILES [#6]-[#7](-[#6])-[#6](=O)-[#6]-[#8]-[#6](=O)-[#6]-c1ccc(-[#8]-[#6](=O)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C20H22N4O5/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22/h3-10H,11-12H2,1-2H3,(H4,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data