

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Homo sapiens (Human)) | BDBM50463145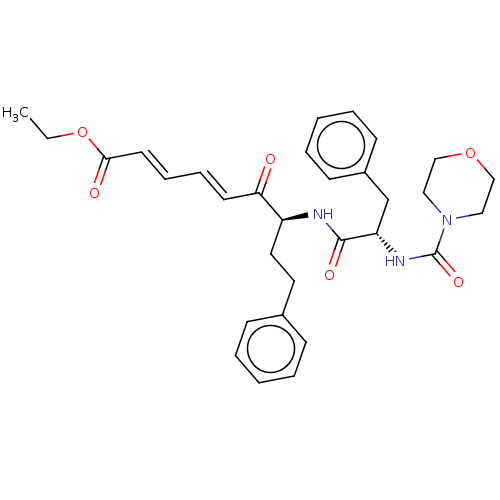 (CHEMBL4244085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463145 (CHEMBL4244085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298372 ((S)-1-((S)-1-(4-(benzyloxy)-3-(trifluoromethyl)ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298372 ((S)-1-((S)-1-(4-(benzyloxy)-3-(trifluoromethyl)ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298373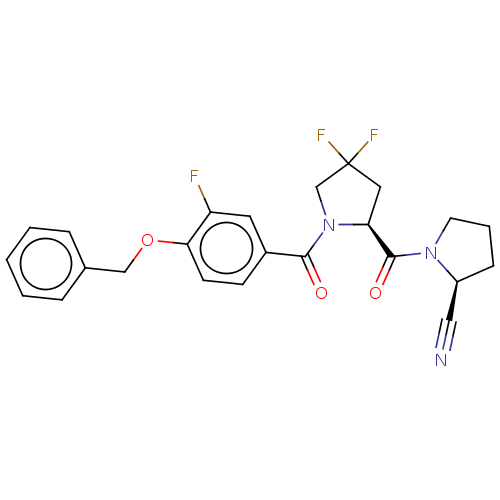 ((S)-1-((S)-1-(4-(benzyloxy)-3-fluorobenzoyl)-4,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298373 ((S)-1-((S)-1-(4-(benzyloxy)-3-fluorobenzoyl)-4,4-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463157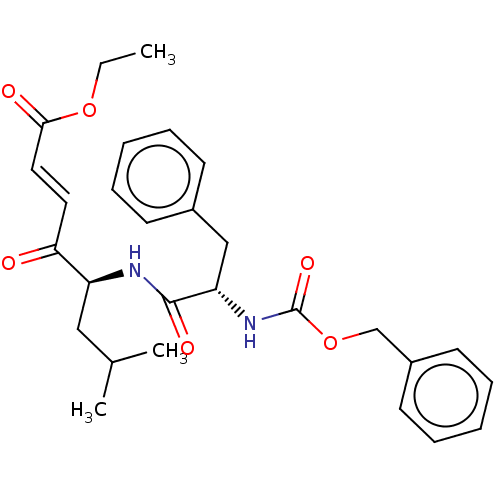 (CHEMBL4244656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463142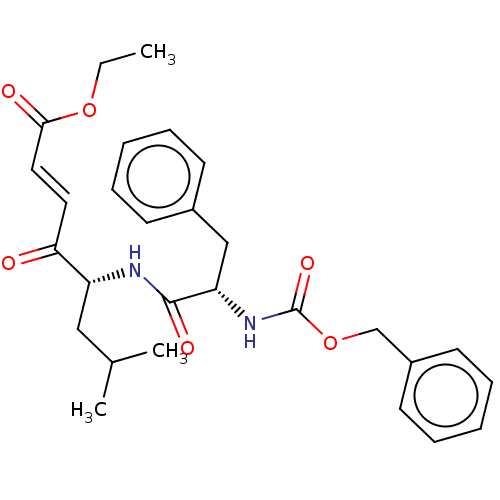 (CHEMBL4238441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298357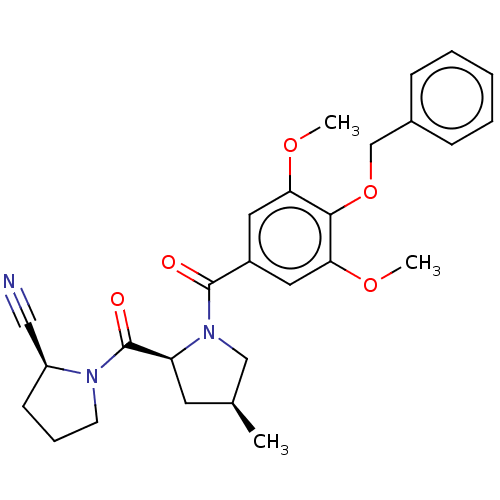 ((S)-1-((2S,4S)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298357 ((S)-1-((2S,4S)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50463157 (CHEMBL4244656) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50463142 (CHEMBL4238441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463157 (CHEMBL4244656) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463154 (CHEMBL4251480) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463142 (CHEMBL4238441) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298355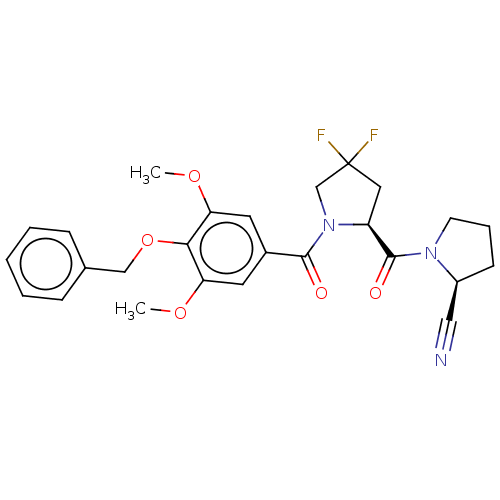 ((S)-1-((2S)-1-(4-(benzyloxy)-3,5-dimethoxybenzoyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 48.7 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298355 ((S)-1-((2S)-1-(4-(benzyloxy)-3,5-dimethoxybenzoyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 48.7 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463143 (CHEMBL4245127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463157 (CHEMBL4244656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463154 (CHEMBL4251480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463142 (CHEMBL4238441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine proteinase falcipain 2a (Plasmodium falciparum) | BDBM50463154 (CHEMBL4251480) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain-2 using Cbz-Phe-Arg-AMC as substrate measured for 15 mins by spectrofluorimetric method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298352 ((S)-1-((2S,4R)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60.4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298352 ((S)-1-((2S,4R)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60.4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298345 ((S)-1-((2S,4R)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63.8 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298345 ((S)-1-((2S,4R)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63.8 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463154 (CHEMBL4251480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463149 (CHEMBL4248915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463152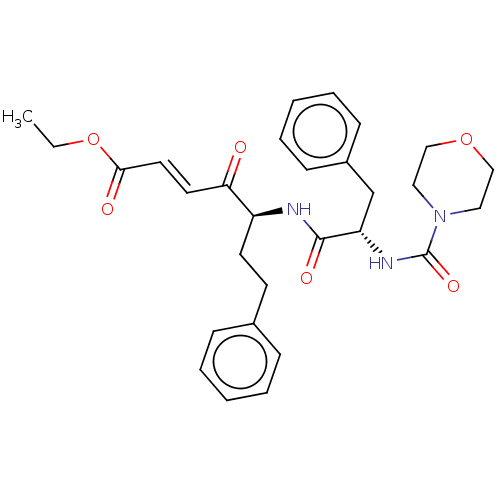 (CHEMBL4242138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463148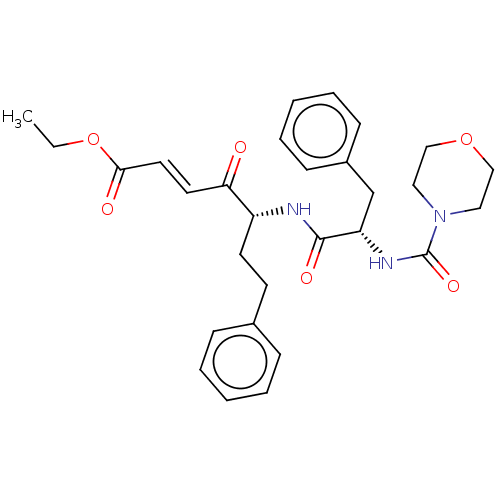 (CHEMBL4247707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50463143 (CHEMBL4245127) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463146 (CHEMBL4240920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463147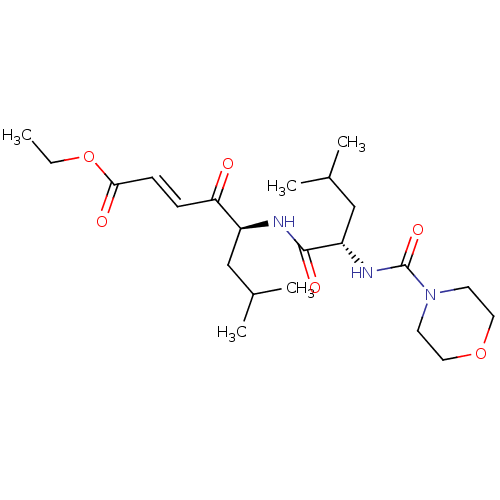 (CHEMBL4237990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463147 (CHEMBL4237990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463147 (CHEMBL4237990) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298363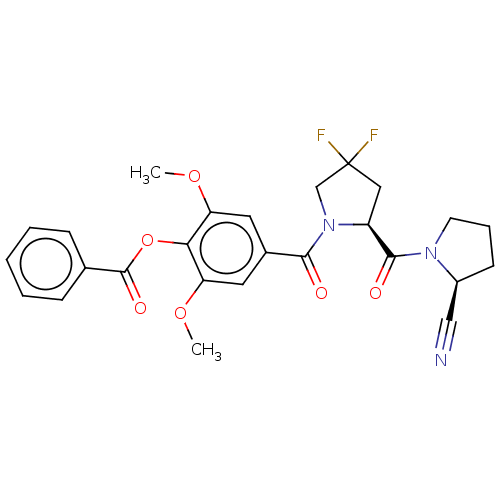 ((S)-1-((2S)-1-(4-benzoyloxy-3,5-dimethoxybenzoyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298363 ((S)-1-((2S)-1-(4-benzoyloxy-3,5-dimethoxybenzoyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463151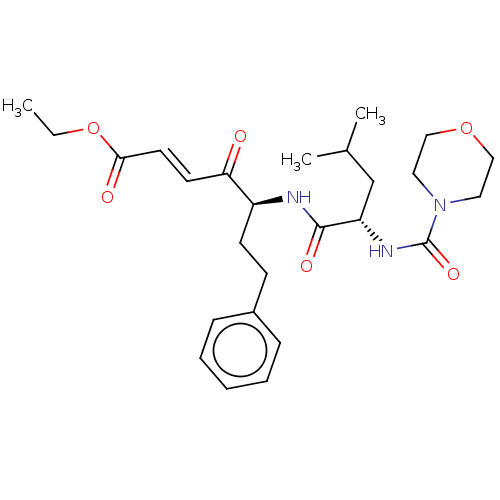 (CHEMBL4249696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50463146 (CHEMBL4240920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463151 (CHEMBL4249696) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463143 (CHEMBL4245127) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463148 (CHEMBL4247707) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50463152 (CHEMBL4242138) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 30 mins by fluorescence method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298356 ((S)-1-((2S,4S)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase-like (Human) | BDBM298359 ((S)-1-((2S,4S)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNACIÓ INSTITUT DE RECERCA BIOMÉDICA; IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10611727 (2020) BindingDB Entry DOI: 10.7270/Q2377CQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298359 ((S)-1-((2S,4S)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM298356 ((S)-1-((2S,4S)-1-(4-(benzyloxy)-3,5-dimethoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITAT DE BARCELONA; FUNDACIO INSTITUT DE RECERCA BIOMEDICA (IRB BARCELONA); IPROTEOS S.L. US Patent | Assay Description POP activity was determined following the method described by Toide et al (Toide K et al., J. Pharmacol. Exp. Ther. 1995; 274:1370-8), using Z-G-P-AM... | US Patent US10125097 (2018) BindingDB Entry DOI: 10.7270/Q22J6DX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50463152 (CHEMBL4242138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine proteinase falcipain 2a (Plasmodium falciparum) | BDBM50463146 (CHEMBL4240920) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain-2 using Cbz-Phe-Arg-AMC as substrate measured for 15 mins by spectrofluorimetric method | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50463147 (CHEMBL4237990) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | Bioorg Med Chem 26: 4624-4634 (2018) Article DOI: 10.1016/j.bmc.2018.07.015 BindingDB Entry DOI: 10.7270/Q29S1TPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |