 Found 74 hits with Last Name = 'takashima' and Initial = 's'
Found 74 hits with Last Name = 'takashima' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50170163
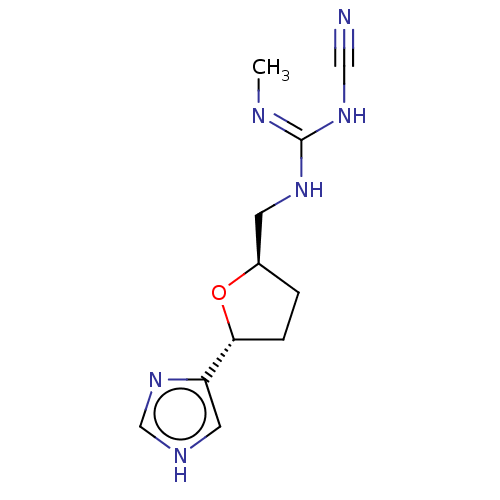
(CHEMBL321860)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474242
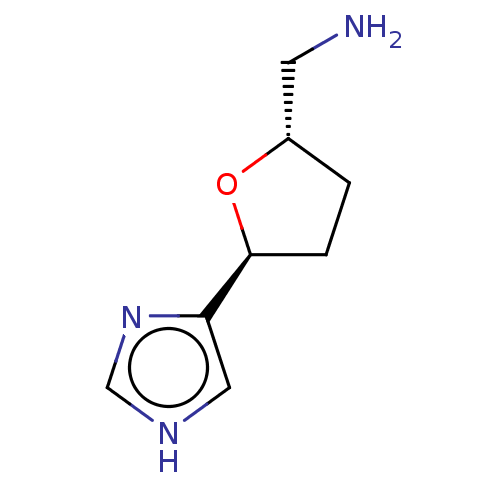
(CHEMBL106931)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474249
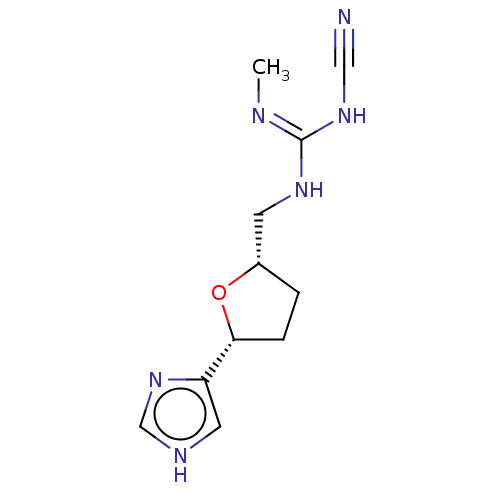
(CHEMBL106158)Show SMILES C\N=C(\NC[C@@H]1CC[C@@H](O1)c1c[nH]cn1)NC#N Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474240
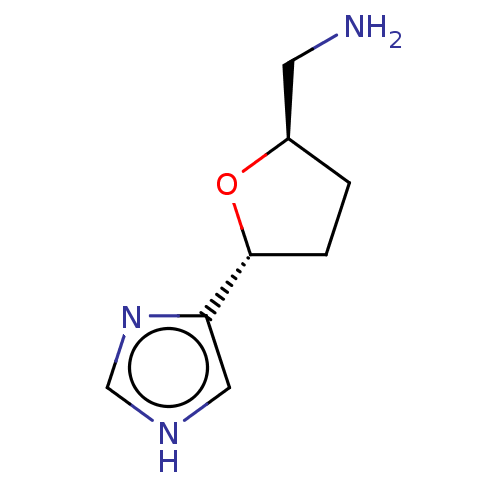
(Imifuramine)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474250
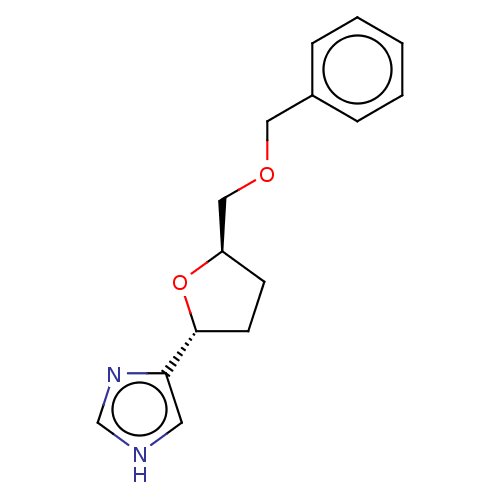
(CHEMBL105803)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474252

(CHEMBL320331)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474252

(CHEMBL320331)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474240

(Imifuramine)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474247
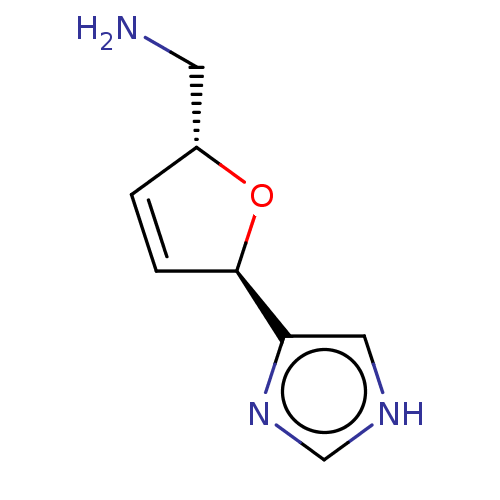
(CHEMBL109130)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474250

(CHEMBL105803)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474244
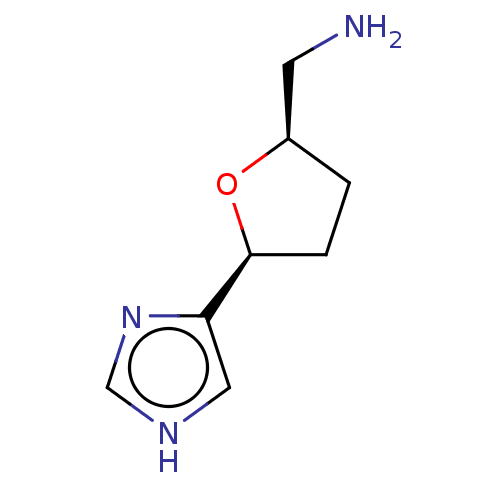
(CHEMBL106913)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474248

(CHEMBL321920)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50170163

(CHEMBL321860)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474248

(CHEMBL321920)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474246
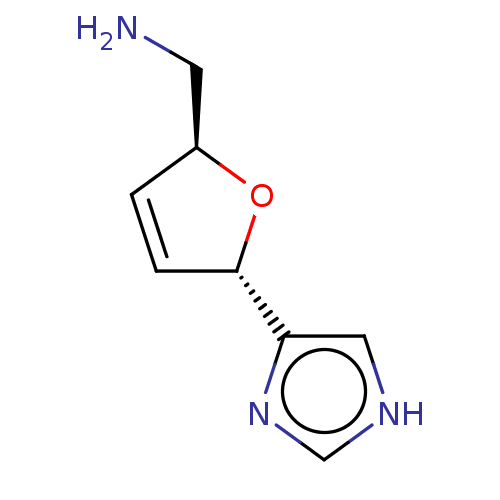
(CHEMBL319658)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474244

(CHEMBL106913)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474253
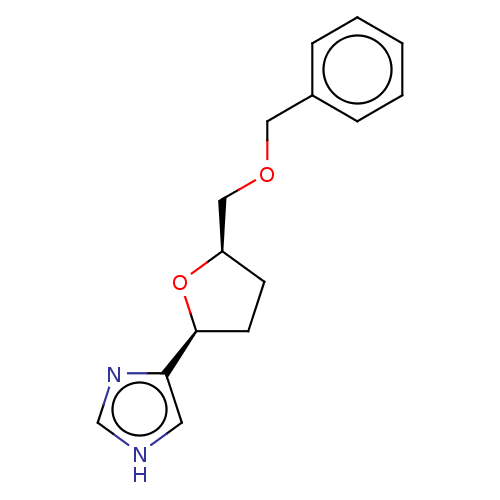
(CHEMBL1632410)Show SMILES C(OCc1ccccc1)[C@H]1CC[C@H](O1)c1c[nH]cn1 |r| Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474249

(CHEMBL106158)Show SMILES C\N=C(\NC[C@@H]1CC[C@@H](O1)c1c[nH]cn1)NC#N Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474251
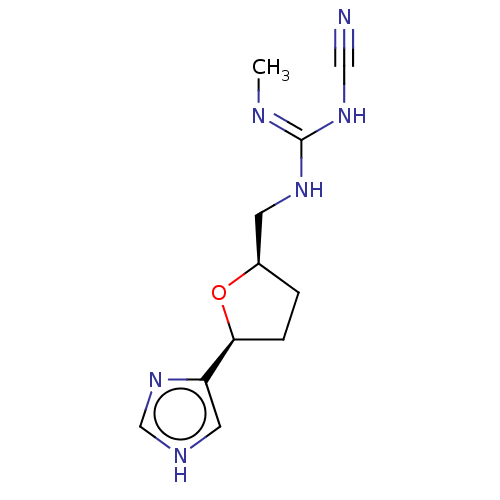
(CHEMBL108649)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474251

(CHEMBL108649)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474241
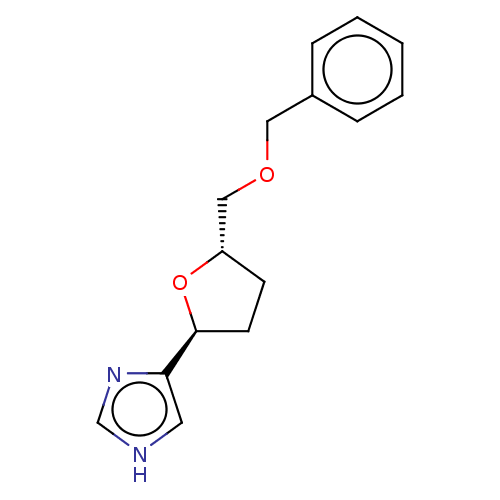
(CHEMBL322256)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Compound was tested for H3 receptor competition binding using [3H]- Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474245
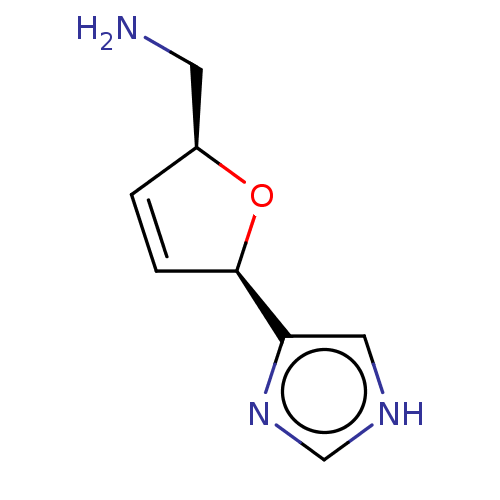
(CHEMBL320279)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474247

(CHEMBL109130)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474239
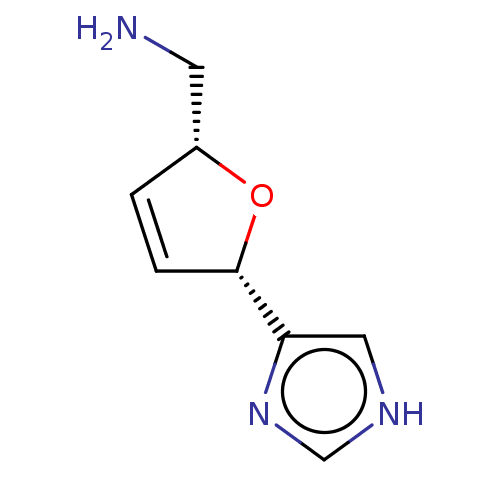
(CHEMBL107877)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474242

(CHEMBL106931)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474253

(CHEMBL1632410)Show SMILES C(OCc1ccccc1)[C@H]1CC[C@H](O1)c1c[nH]cn1 |r| Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474243
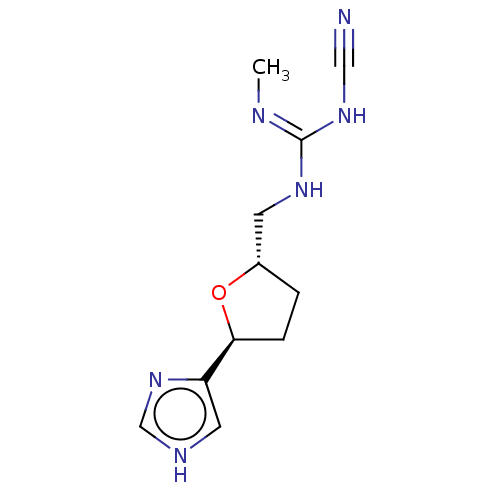
(CHEMBL106570)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474239

(CHEMBL107877)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Compound was tested for H3 receptor competition binding using [3H]- Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474243

(CHEMBL106570)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474241

(CHEMBL322256)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474245

(CHEMBL320279)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474246

(CHEMBL319658)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H4 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Dynein light chain 2, cytoplasmic
(Human) | BDBM528572
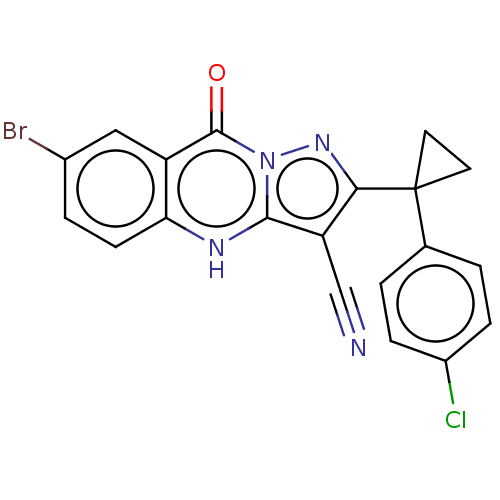
(US11192893, Example 12)Show SMILES Clc1ccc(cc1)C1(CC1)c1nn2c([nH]c3ccc(Br)cc3c2=O)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22J6G18 |
More data for this
Ligand-Target Pair | |
Dynein light chain 2, cytoplasmic
(Human) | BDBM528573

(US11192893, Example 27)Show SMILES Clc1ccc(cc1)C1CCC(CC1)c1nn2c([nH]c3ccccc3c2=O)c1C#N |(9.24,.55,;7.7,.55,;6.93,-.79,;5.39,-.79,;4.62,.55,;5.39,1.88,;6.93,1.88,;3.08,.55,;2.31,-.79,;.77,-.79,;0,.55,;.77,1.88,;2.31,1.88,;-1.54,.55,;-2.44,1.79,;-3.91,1.32,;-3.91,-.22,;-5.24,-.99,;-6.57,-.22,;-7.91,-.99,;-9.24,-.22,;-9.24,1.32,;-7.91,2.09,;-6.57,1.32,;-5.24,2.09,;-5.24,3.63,;-2.44,-.7,;-1.97,-2.16,;-1.49,-3.63,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22J6G18 |
More data for this
Ligand-Target Pair | |
Dynein light chain 2, cytoplasmic
(Human) | BDBM528576
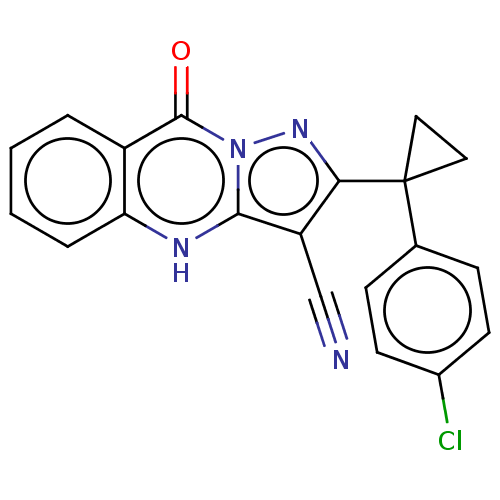
(US11192893, Example 31)Show SMILES Clc1ccc(cc1)C1(CC1)c1nn2c([nH]c3ccccc3c2=O)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22J6G18 |
More data for this
Ligand-Target Pair | |
Dynein light chain 2, cytoplasmic
(Human) | BDBM528579

(US11192893, Example 29)Show SMILES Clc1ccc(OCCCc2nn3c([nH]c4ccccc4c3=O)c2C#N)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22J6G18 |
More data for this
Ligand-Target Pair | |
Dynein light chain 2, cytoplasmic
(Human) | BDBM528571

(US11192893, Example 5)Show SMILES Clc1ccc(cc1)C1(CC1)c1nn2c([nH]c(=O)c3cc(I)ccc23)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22J6G18 |
More data for this
Ligand-Target Pair | |
Dynein light chain 2, cytoplasmic
(Human) | BDBM528570
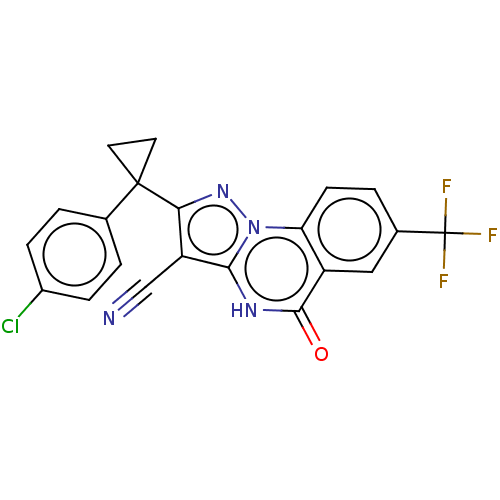
(US11192893, Example 4)Show SMILES FC(F)(F)c1ccc2c(c1)c(=O)[nH]c1c(C#N)c(nn21)C1(CC1)c1ccc(Cl)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22J6G18 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474241

(CHEMBL322256)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474247

(CHEMBL109130)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 269 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H3 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474244

(CHEMBL106913)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM81542
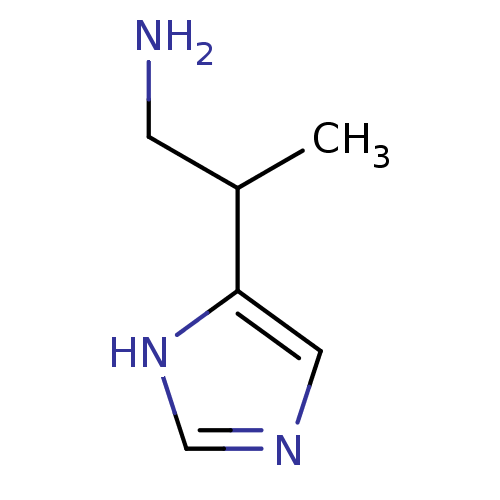
(Beta-Methylhistamine | CAS_565544 | NSC_565544)Show InChI InChI=1S/C6H11N3/c1-5(2-7)6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H3 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50170163

(CHEMBL321860)Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474249

(CHEMBL106158)Show SMILES C\N=C(\NC[C@@H]1CC[C@@H](O1)c1c[nH]cn1)NC#N Show InChI InChI=1S/C11H16N6O/c1-13-11(16-6-12)15-4-8-2-3-10(18-8)9-5-14-7-17-9/h5,7-8,10H,2-4H2,1H3,(H,14,17)(H2,13,15,16)/t8-,10+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 224 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474248

(CHEMBL321920)Show InChI InChI=1S/C8H13N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h4-6,8H,1-3,9H2,(H,10,11)/t6-,8+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474250

(CHEMBL105803)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 91 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H3 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50474252

(CHEMBL320331)Show InChI InChI=1S/C15H18N2O2/c1-2-4-12(5-3-1)9-18-10-13-6-7-15(19-13)14-8-16-11-17-14/h1-5,8,11,13,15H,6-7,9-10H2,(H,16,17)/t13-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 191 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H3 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50474246

(CHEMBL319658)Show InChI InChI=1S/C8H11N3O/c9-3-6-1-2-8(12-6)7-4-10-5-11-7/h1-2,4-6,8H,3,9H2,(H,10,11)/t6-,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.51E+4 | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of the forskolin-stimulated cAMP production in SK-N-MC cells expressing human Histamine H4 receptor |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data