

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 10 mins by stopped flow assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50276359 (CHEMBL4129303) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate after 15 mins by amplex red reagent based assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50276368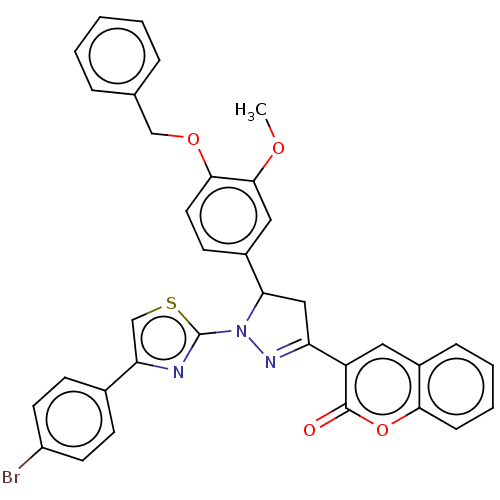 (CHEMBL4128371) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579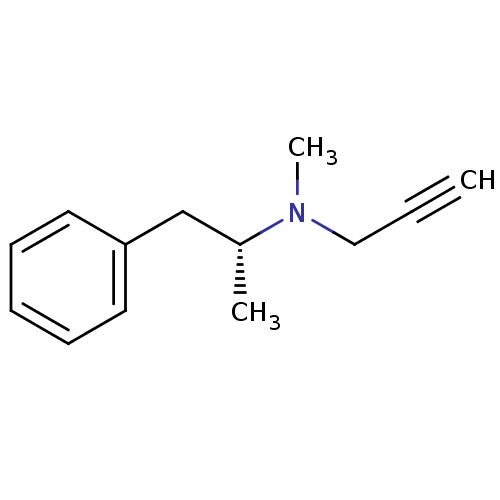 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate after 15 mins by amplex red reagent based assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193852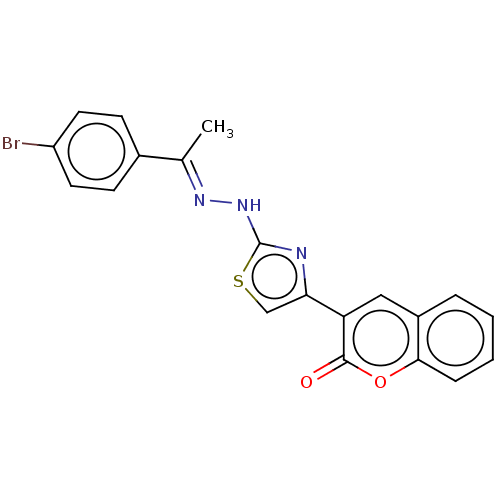 ((E)-3-(2-(2-(1-(4-bromophenyl)ethylidene)hydraziny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276360 (CHEMBL4126559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193866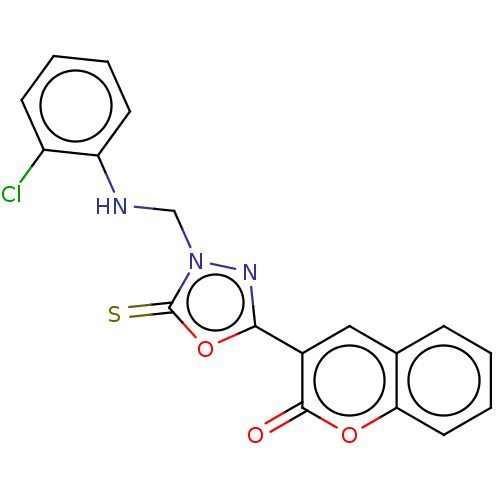 (3-(4-((2-Chlorophenylamino)methyl)-5-thioxo-4,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276310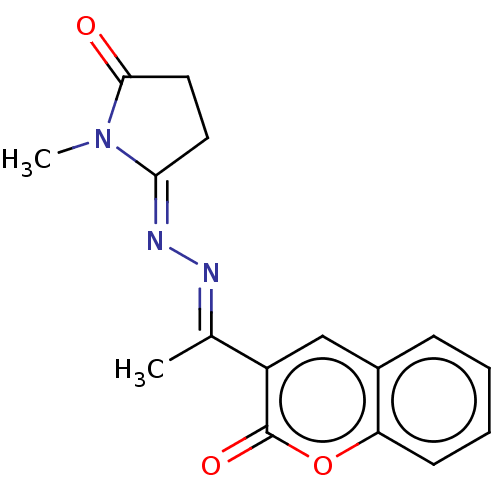 (CHEMBL4127794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276366 (CHEMBL4129984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276369 (CHEMBL4127948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193869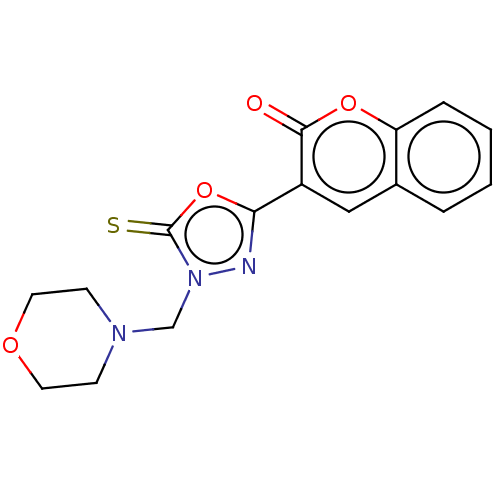 (3-(4-(Morpholinomethyl)-5-thioxo-4,5-dihydro-1,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276367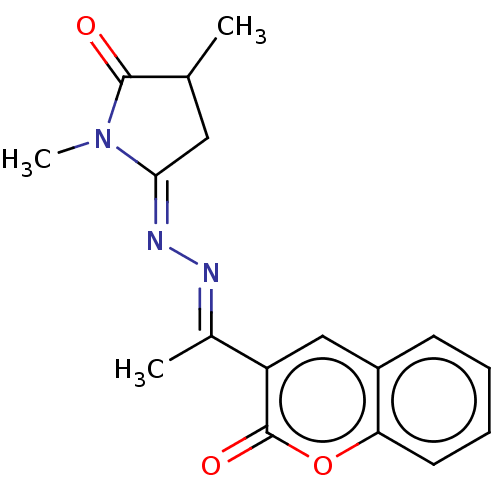 (CHEMBL4129191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193860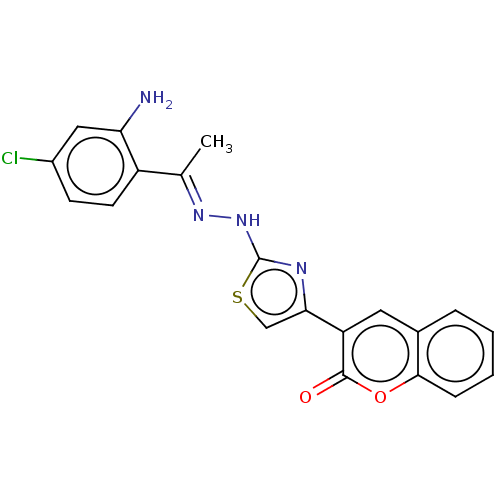 ((E)-3-(2-(2-((2-amino-4-chlorophenyl)(phenyl)methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of bovine kidney ALR1 using D-glucoronic acid as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50276361 (CHEMBL4129493) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf intestinal alkaline phosphatase using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after 30 ... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193864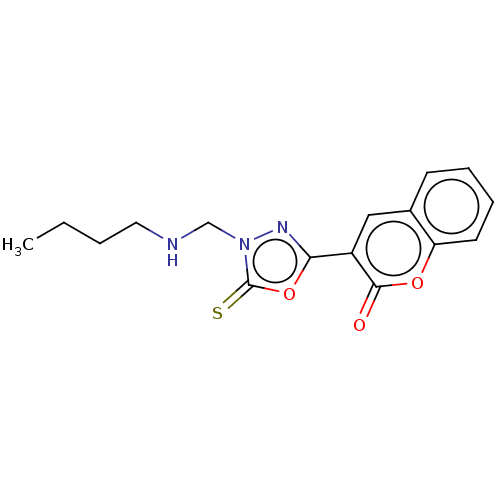 (3-(4-((Butylamino)methyl)-5-thioxo-4,5-dihydro-1,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of bovine kidney ALR1 using D-glucoronic acid as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM11682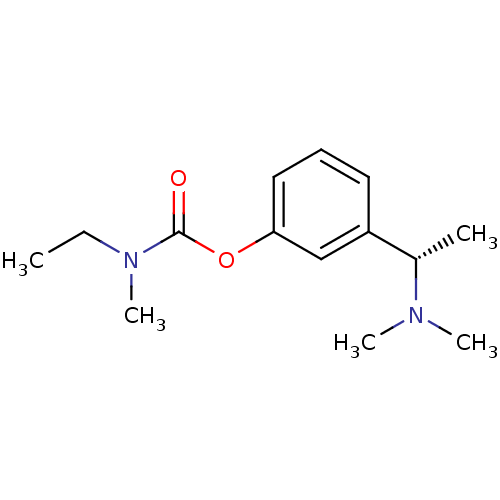 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152478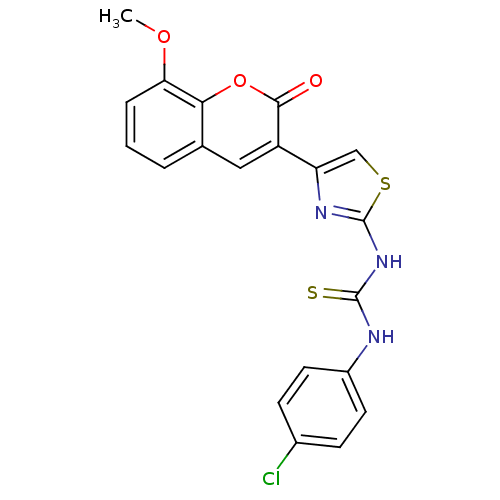 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM152467 (1-(4-Fluorophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50276371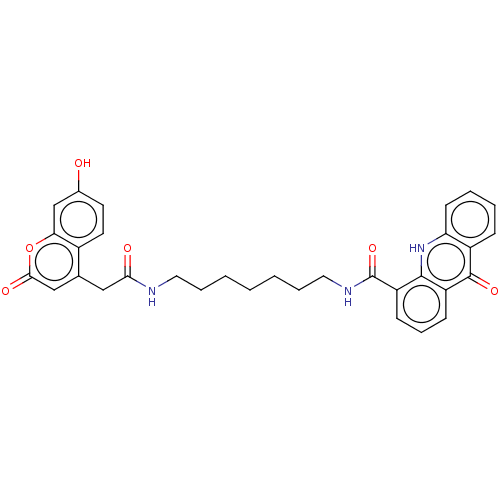 (CHEMBL4128061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured at 1 min ti... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50276365 (CHEMBL4127281) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50327939 (7-methoxytacrine | CHEMBL1256415) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured at 1 min ti... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467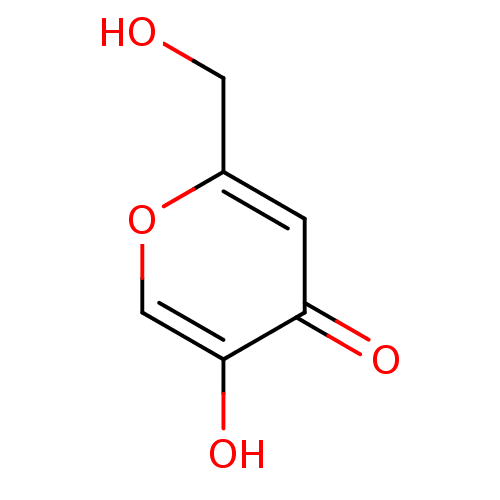 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50276370 (CHEMBL4126039) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM32020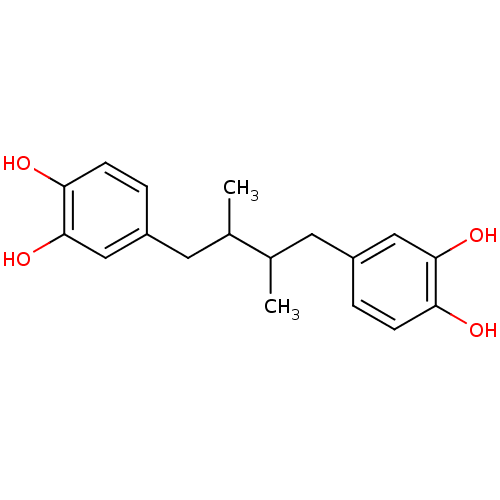 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50276371 (CHEMBL4128061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human plasmatic BuChE using butylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured at 1 min time... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50276360 (CHEMBL4126559) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50276369 (CHEMBL4127948) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50276366 (CHEMBL4129984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50276367 (CHEMBL4129191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50276310 (CHEMBL4127794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||