 Found 78 hits with Last Name = 'verhelst' and Initial = 'shl'
Found 78 hits with Last Name = 'verhelst' and Initial = 'shl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mucosa-associated lymphoid tissue lymphoma translocation protein 1
(Homo sapiens (Human)) | BDBM50514789
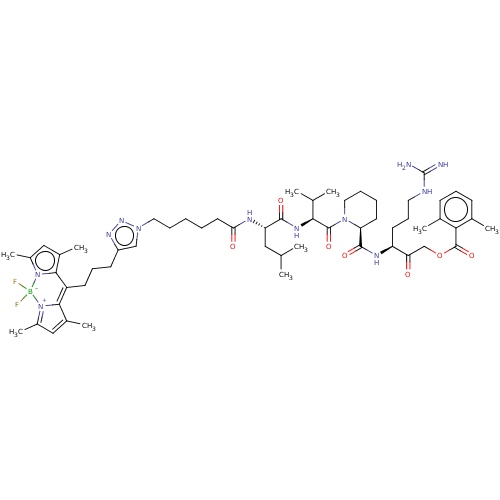
(CHEMBL4564966)Show SMILES CC(C)C[C@H](NC(=O)CCCCCn1cc(CCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)nn1)C(=O)N[C@@H](C(C)C)C(=O)N1CCCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C |r,c:19,22,25| Show InChI InChI=1S/C57H83BF2N12O7/c1-34(2)29-45(64-48(74)25-12-11-14-27-69-32-42(67-68-69)21-17-22-43-51-38(7)30-40(9)71(51)58(59,60)72-41(10)31-39(8)52(43)72)53(75)66-50(35(3)4)55(77)70-28-15-13-24-46(70)54(76)65-44(23-18-26-63-57(61)62)47(73)33-79-56(78)49-36(5)19-16-20-37(49)6/h16,19-20,30-32,34-35,44-46,50H,11-15,17-18,21-29,33H2,1-10H3,(H,64,74)(H,65,76)(H,66,75)(H4,61,62,63)/t44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)... |
J Med Chem 63: 3996-4004 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01879
BindingDB Entry DOI: 10.7270/Q2SN0DBR |
More data for this
Ligand-Target Pair | |
Mucosa-associated lymphoid tissue lymphoma translocation protein 1
(Homo sapiens (Human)) | BDBM50514790
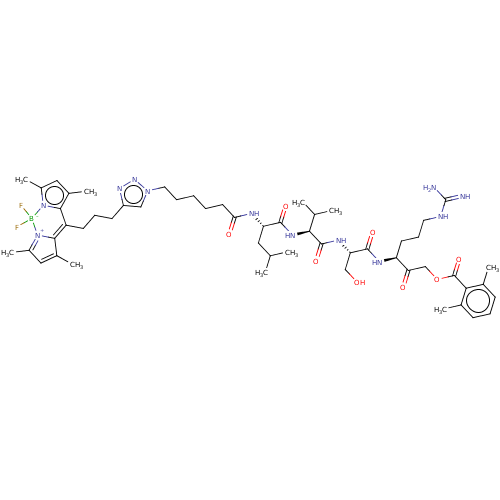
(CHEMBL4565998)Show SMILES CC(C)C[C@H](NC(=O)CCCCCn1cc(CCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)nn1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C |r,c:19,22,25| Show InChI InChI=1S/C54H79BF2N12O8/c1-31(2)25-42(61-45(72)22-12-11-13-24-67-28-39(65-66-67)19-15-20-40-48-35(7)26-37(9)68(48)55(56,57)69-38(10)27-36(8)49(40)69)50(73)64-47(32(3)4)52(75)63-43(29-70)51(74)62-41(21-16-23-60-54(58)59)44(71)30-77-53(76)46-33(5)17-14-18-34(46)6/h14,17-18,26-28,31-32,41-43,47,70H,11-13,15-16,19-25,29-30H2,1-10H3,(H,61,72)(H,62,74)(H,63,75)(H,64,73)(H4,58,59,60)/t41-,42-,43-,47-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)... |
J Med Chem 63: 3996-4004 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01879
BindingDB Entry DOI: 10.7270/Q2SN0DBR |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50514790

(CHEMBL4565998)Show SMILES CC(C)C[C@H](NC(=O)CCCCCn1cc(CCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)nn1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C |r,c:19,22,25| Show InChI InChI=1S/C54H79BF2N12O8/c1-31(2)25-42(61-45(72)22-12-11-13-24-67-28-39(65-66-67)19-15-20-40-48-35(7)26-37(9)68(48)55(56,57)69-38(10)27-36(8)49(40)69)50(73)64-47(32(3)4)52(75)63-43(29-70)51(74)62-41(21-16-23-60-54(58)59)44(71)30-77-53(76)46-33(5)17-14-18-34(46)6/h14,17-18,26-28,31-32,41-43,47,70H,11-13,15-16,19-25,29-30H2,1-10H3,(H,61,72)(H,62,74)(H,63,75)(H,64,73)(H4,58,59,60)/t41-,42-,43-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ... |
J Med Chem 63: 3996-4004 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01879
BindingDB Entry DOI: 10.7270/Q2SN0DBR |
More data for this
Ligand-Target Pair | |
Mucosa-associated lymphoid tissue lymphoma translocation protein 1
(Homo sapiens (Human)) | BDBM50514791
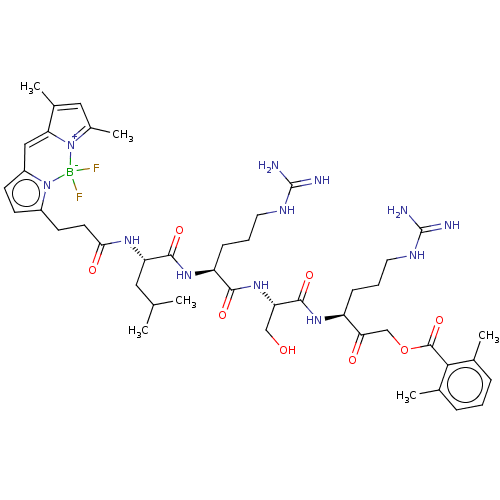
(CHEMBL3814336)Show SMILES CC(C)C[C@H](NC(=O)CCc1ccc2C=C3C(C)=CC(C)=[N+]3[B-](F)(F)n12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C |r,c:17,20,t:14| Show InChI InChI=1S/C45H65BF2N12O8/c1-25(2)20-34(55-38(63)17-16-30-14-15-31-22-36-28(5)21-29(6)59(36)46(47,48)60(30)31)41(65)57-33(13-9-19-54-45(51)52)40(64)58-35(23-61)42(66)56-32(12-8-18-53-44(49)50)37(62)24-68-43(67)39-26(3)10-7-11-27(39)4/h7,10-11,14-15,21-22,25,32-35,61H,8-9,12-13,16-20,23-24H2,1-6H3,(H,55,63)(H,56,66)(H,57,65)(H,58,64)(H4,49,50,53)(H4,51,52,54)/t32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)... |
J Med Chem 63: 3996-4004 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01879
BindingDB Entry DOI: 10.7270/Q2SN0DBR |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50514791

(CHEMBL3814336)Show SMILES CC(C)C[C@H](NC(=O)CCc1ccc2C=C3C(C)=CC(C)=[N+]3[B-](F)(F)n12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C |r,c:17,20,t:14| Show InChI InChI=1S/C45H65BF2N12O8/c1-25(2)20-34(55-38(63)17-16-30-14-15-31-22-36-28(5)21-29(6)59(36)46(47,48)60(30)31)41(65)57-33(13-9-19-54-45(51)52)40(64)58-35(23-61)42(66)56-32(12-8-18-53-44(49)50)37(62)24-68-43(67)39-26(3)10-7-11-27(39)4/h7,10-11,14-15,21-22,25,32-35,61H,8-9,12-13,16-20,23-24H2,1-6H3,(H,55,63)(H,56,66)(H,57,65)(H,58,64)(H4,49,50,53)(H4,51,52,54)/t32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ... |
J Med Chem 63: 3996-4004 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01879
BindingDB Entry DOI: 10.7270/Q2SN0DBR |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50544647

(CHEMBL4634122)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)Oc1ccccc1 Show InChI InChI=1S/C24H26NO5P/c1-19(2)23(25-24(26)28-18-20-12-6-3-7-13-20)31(27,29-21-14-8-4-9-15-21)30-22-16-10-5-11-17-22/h3-17,19,23H,18H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using flurogenic substrate as elastase substrate V by fluorescence assay |
ACS Med Chem Lett 11: 1739-1744 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00284
BindingDB Entry DOI: 10.7270/Q29P357N |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50514789

(CHEMBL4564966)Show SMILES CC(C)C[C@H](NC(=O)CCCCCn1cc(CCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)nn1)C(=O)N[C@@H](C(C)C)C(=O)N1CCCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C |r,c:19,22,25| Show InChI InChI=1S/C57H83BF2N12O7/c1-34(2)29-45(64-48(74)25-12-11-14-27-69-32-42(67-68-69)21-17-22-43-51-38(7)30-40(9)71(51)58(59,60)72-41(10)31-39(8)52(43)72)53(75)66-50(35(3)4)55(77)70-28-15-13-24-46(70)54(76)65-44(23-18-26-63-57(61)62)47(73)33-79-56(78)49-36(5)19-16-20-37(49)6/h16,19-20,30-32,34-35,44-46,50H,11-15,17-18,21-29,33H2,1-10H3,(H,64,74)(H,65,76)(H,66,75)(H4,61,62,63)/t44-,45-,46-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ... |
J Med Chem 63: 3996-4004 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01879
BindingDB Entry DOI: 10.7270/Q2SN0DBR |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50544646

(CHEMBL4635621)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H26NO4P/c1-19(2)23(25-24(26)28-18-20-12-6-3-7-13-20)30(27,22-16-10-5-11-17-22)29-21-14-8-4-9-15-21/h3-17,19,23H,18H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human proteinase 3 using flurogenic substrate as elastase substrate V by fluorescence assay |
ACS Med Chem Lett 11: 1739-1744 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00284
BindingDB Entry DOI: 10.7270/Q29P357N |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50544646

(CHEMBL4635621)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H26NO4P/c1-19(2)23(25-24(26)28-18-20-12-6-3-7-13-20)30(27,22-16-10-5-11-17-22)29-21-14-8-4-9-15-21/h3-17,19,23H,18H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using flurogenic substrate as elastase substrate V by fluorescence assay |
ACS Med Chem Lett 11: 1739-1744 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00284
BindingDB Entry DOI: 10.7270/Q29P357N |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Homo sapiens (Human)) | BDBM50544648

(CHEMBL4636971)Show SMILES CCCP(=O)(Oc1ccccc1)C(NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C21H28NO4P/c1-4-15-27(24,26-19-13-9-6-10-14-19)20(17(2)3)22-21(23)25-16-18-11-7-5-8-12-18/h5-14,17,20H,4,15-16H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human proteinase 3 using flurogenic substrate as elastase substrate V by fluorescence assay |
ACS Med Chem Lett 11: 1739-1744 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00284
BindingDB Entry DOI: 10.7270/Q29P357N |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591337
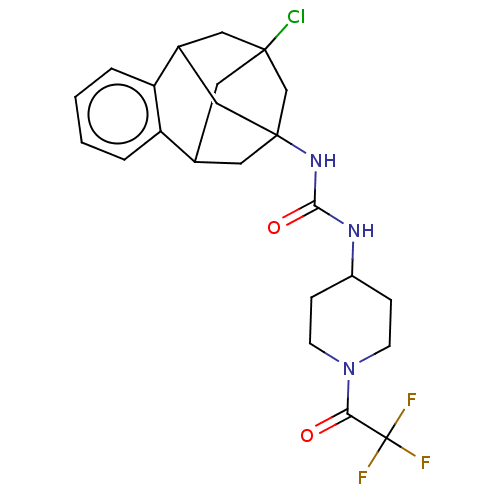
(CHEMBL5192445)Show SMILES FC(F)(F)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591341
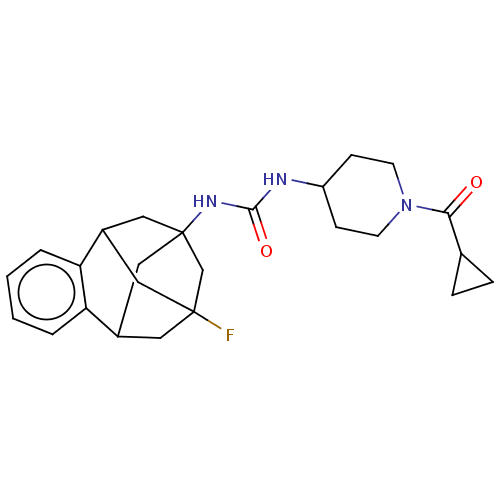
(CHEMBL5177372)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591344
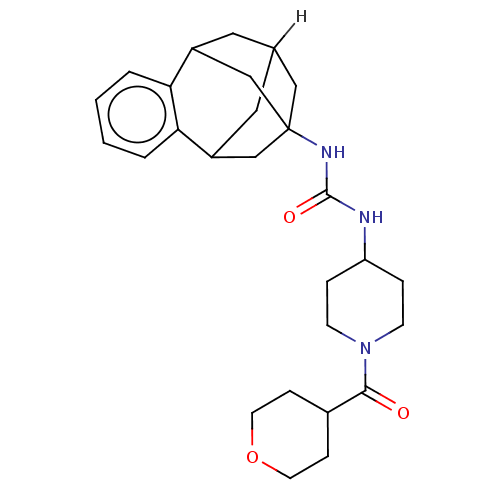
(CHEMBL5208857)Show SMILES [2H]C12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591337

(CHEMBL5192445)Show SMILES FC(F)(F)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591340
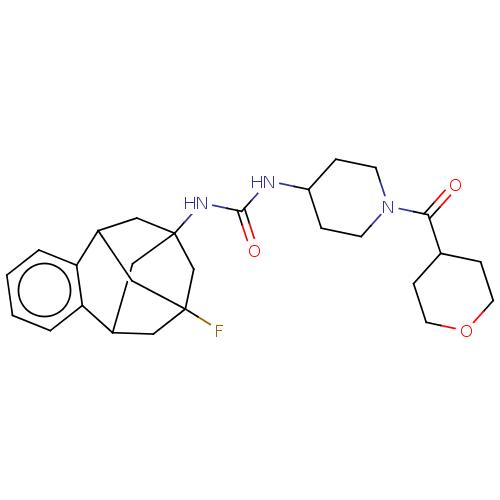
(CHEMBL5203787)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591341

(CHEMBL5177372)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591336

(CHEMBL5179027)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591334
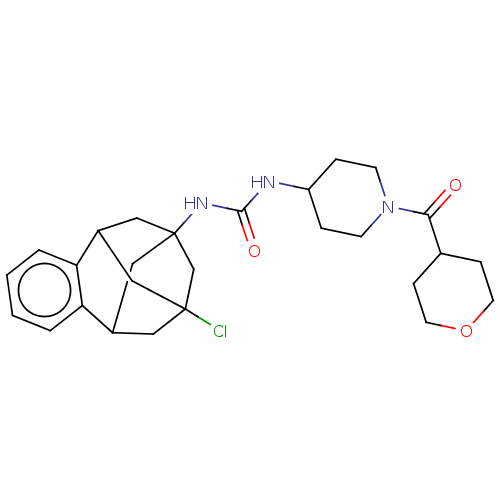
(CHEMBL5191146)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
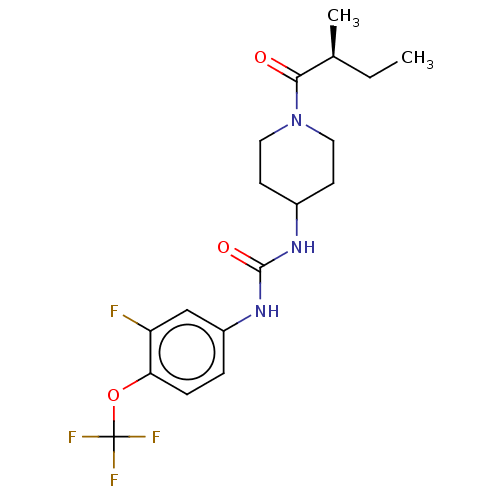
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM158481

(US9029401, 1728 (t-TUCB))Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 |r,wU:8.7,wD:11.14,(10,,;8.67,-.77,;8.67,-2.31,;7.34,,;6,-.77,;4.67,,;4.67,1.54,;3.33,2.31,;2,1.54,;2,,;.67,-.77,;-.67,,;-.67,1.54,;.67,2.31,;-2,-.77,;-3.33,,;-3.33,1.54,;-4.67,-.77,;-6,,;-6,1.54,;-7.34,2.31,;-8.67,1.54,;-10,2.31,;-10,3.85,;-10,5.39,;-8.67,4.62,;-11.34,4.62,;-8.67,,;-7.34,-.77,;6,2.31,;7.34,1.54,)| Show InChI InChI=1S/C21H21F3N2O5/c22-21(23,24)31-18-11-5-15(6-12-18)26-20(29)25-14-3-9-17(10-4-14)30-16-7-1-13(2-8-16)19(27)28/h1-2,5-8,11-12,14,17H,3-4,9-10H2,(H,27,28)(H2,25,26,29)/t14-,17- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591327
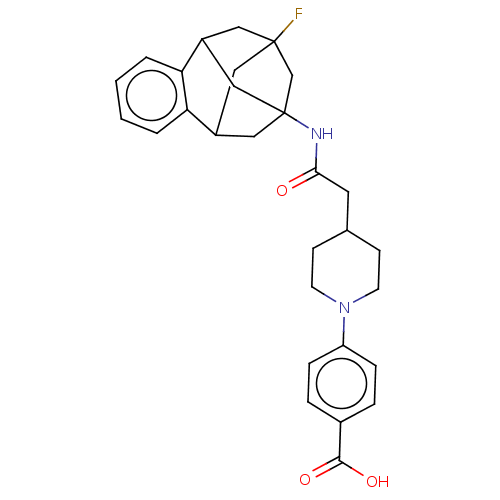
(CHEMBL5177544)Show SMILES OC(=O)c1ccc(cc1)N1CCC(CC(=O)NC23CC4CC(F)(CC(C2)c2ccccc42)C3)CC1 |TLB:31:19:24.25.23:32,26:24:18.19.20:32,27:26:25:21.23.32,22:21:25:31.26.18.19,THB:20:19:25:21.23.32,20:21:25:31.26.18.19| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591336

(CHEMBL5179027)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591331
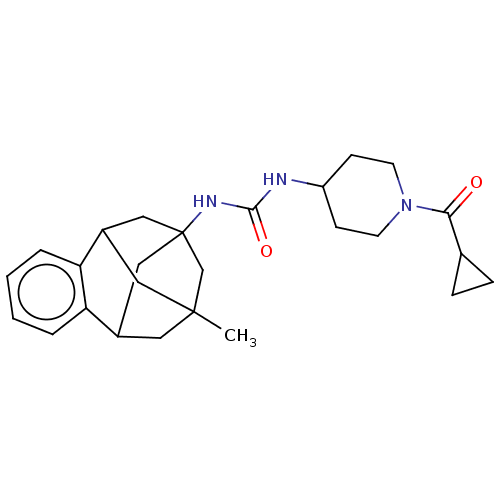
(CHEMBL5197282)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591330

(CHEMBL5197431)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591331

(CHEMBL5197282)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591344

(CHEMBL5208857)Show SMILES [2H]C12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591345

(CHEMBL5196519)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)CCC1(CCC#C)N=N1 |c:40,TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591345

(CHEMBL5196519)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)CCC1(CCC#C)N=N1 |c:40,TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591340

(CHEMBL5203787)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217448
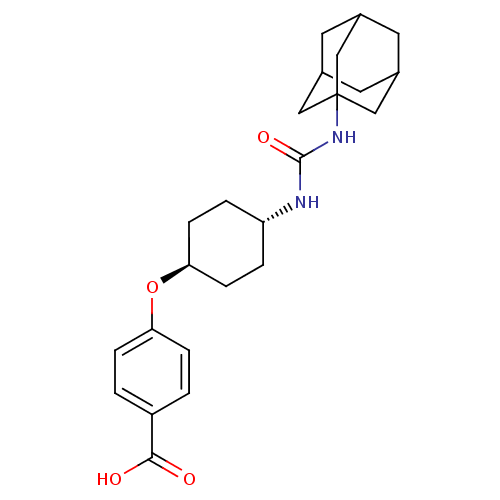
(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591335

(CHEMBL5179500)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591333
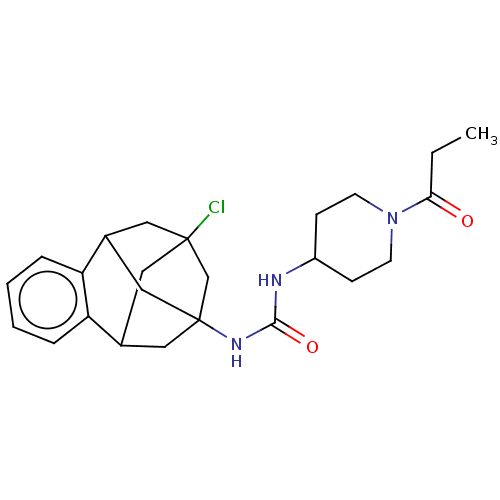
(CHEMBL5197313)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:28:16:21.22.20:29,23:21:16.17.15:29,24:23:22:18.20.29,19:18:22:28.23.16.15,THB:17:16:22:18.20.29,17:18:22:28.23.16.15| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591338

(CHEMBL5203245)Show SMILES FC1(CC1)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591332
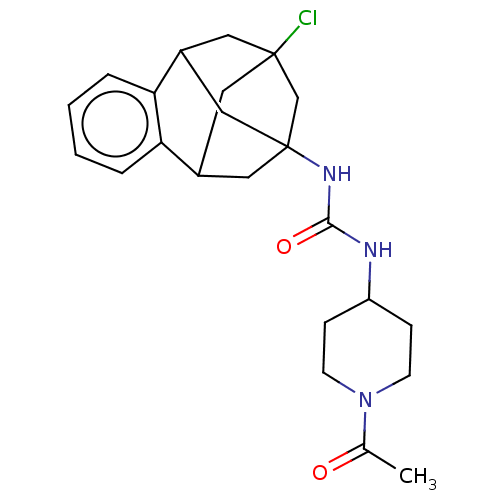
(CHEMBL5195267)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:27:15:20.21.19:28,22:20:15.16.14:28,23:22:21:17.19.28,18:17:21:27.22.15.14,THB:16:15:21:17.19.28,16:17:21:27.22.15.14| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591338

(CHEMBL5203245)Show SMILES FC1(CC1)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591328
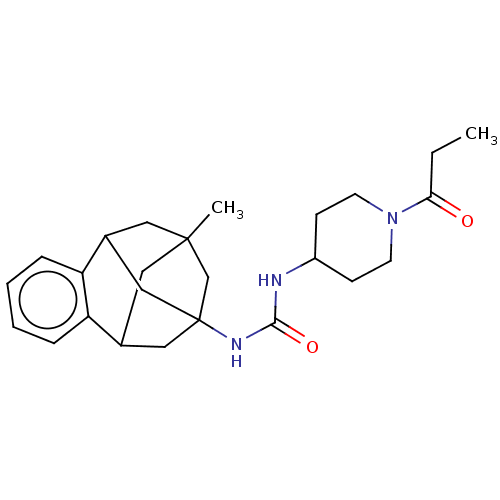
(CHEMBL5193653)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:28:16:21.22.20:29,23:21:16.17.15:29,24:23:22:18.20.29,19:18:22:28.23.16.15,THB:17:16:22:18.20.29,17:18:22:28.23.16.15| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591333

(CHEMBL5197313)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:28:16:21.22.20:29,23:21:16.17.15:29,24:23:22:18.20.29,19:18:22:28.23.16.15,THB:17:16:22:18.20.29,17:18:22:28.23.16.15| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591334

(CHEMBL5191146)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591343
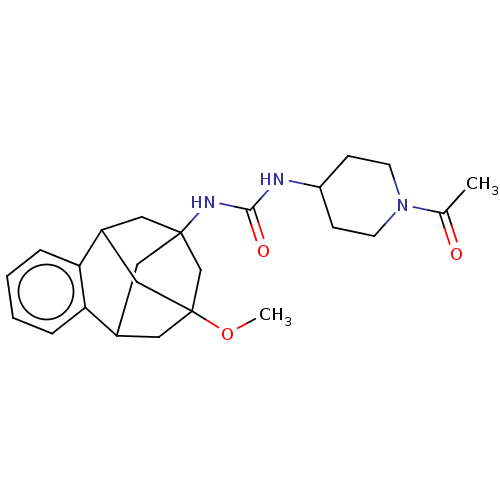
(CHEMBL5209279)Show SMILES COC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(C)=O |TLB:10:8:4.5.3:16,15:4:8.9.7:16,14:15:5:2.3.16,1:2:5:10.15.8.7,THB:9:8:5:2.3.16,9:2:5:10.15.8.7| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591329
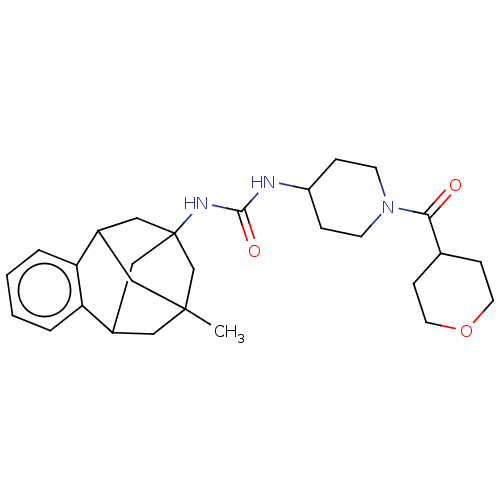
(CHEMBL5179393)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591335

(CHEMBL5179500)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591332

(CHEMBL5195267)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:27:15:20.21.19:28,22:20:15.16.14:28,23:22:21:17.19.28,18:17:21:27.22.15.14,THB:16:15:21:17.19.28,16:17:21:27.22.15.14| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591329

(CHEMBL5179393)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581722
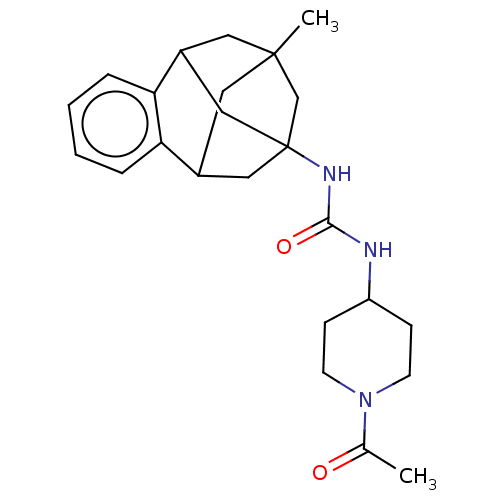
(CHEMBL5081815)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:27:15:20.21.19:28,23:22:14:16.17.28,26:27:14:16.17.28,THB:19:20:14:16.17.28,19:17:14:27.20.21.22,22:20:15.14.16:28,18:17:14:27.20.21.22| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50581722

(CHEMBL5081815)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:27:15:20.21.19:28,23:22:14:16.17.28,26:27:14:16.17.28,THB:19:20:14:16.17.28,19:17:14:27.20.21.22,22:20:15.14.16:28,18:17:14:27.20.21.22| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591330

(CHEMBL5197431)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854
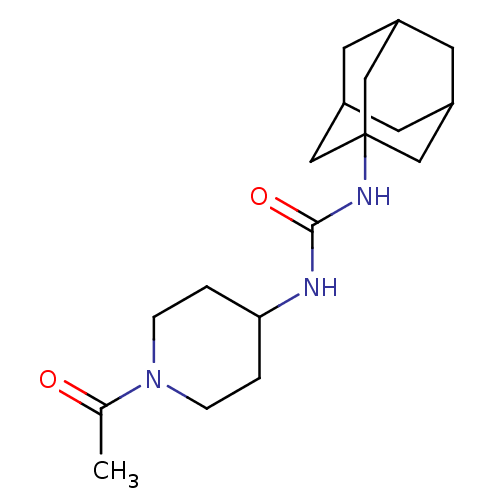
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591339
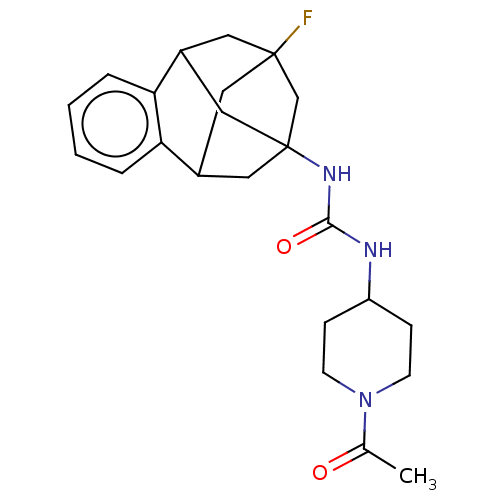
(CHEMBL5169759)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(F)(CC(C1)c1ccccc31)C2 |TLB:27:15:20.21.19:28,22:20:15.16.14:28,23:22:21:17.19.28,18:17:21:27.22.15.14,THB:16:15:21:17.19.28,16:17:21:27.22.15.14| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591328

(CHEMBL5193653)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:28:16:21.22.20:29,23:21:16.17.15:29,24:23:22:18.20.29,19:18:22:28.23.16.15,THB:17:16:22:18.20.29,17:18:22:28.23.16.15| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 2
(Mus musculus) | BDBM50547464
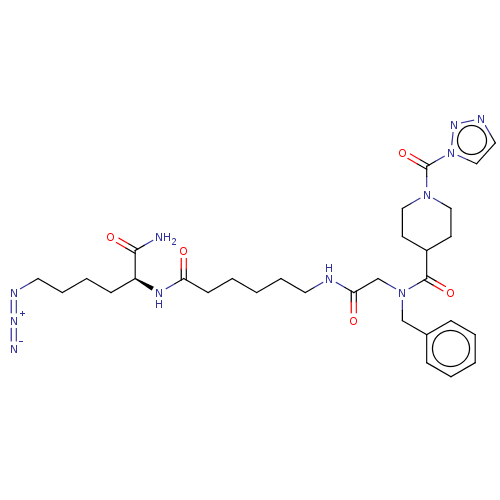
(CHEMBL4776695)Show SMILES NC(=O)[C@H](CCCCN=[N+]=[N-])NC(=O)CCCCCNC(=O)CN(Cc1ccccc1)C(=O)C1CCN(CC1)C(=O)n1ccnn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FP-Rh binding to APT-1/2 in mouse brain cytosolic fraction preincubated for 1 hr followed by FP-Rh addition and measured after 1 hr by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01043
BindingDB Entry DOI: 10.7270/Q2NZ8C8B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data