

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50116538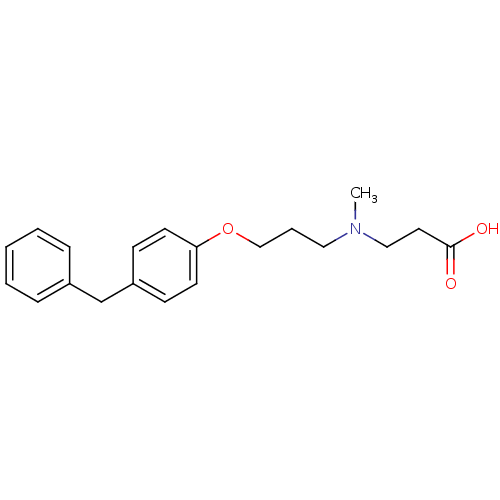 (3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Non-competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50197085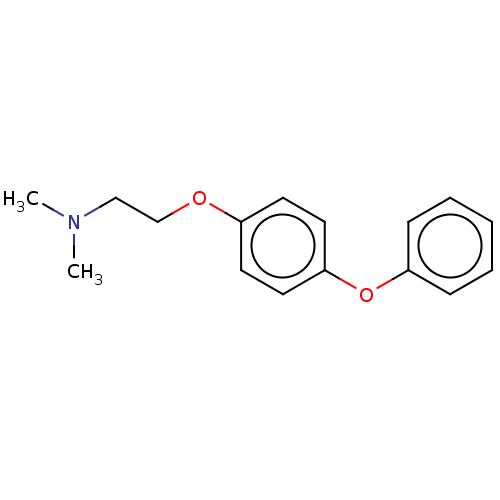 (CHEMBL3921982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Binding affinity to beta1 adrenergic receptor (unknown origin) by radioligand binding assay | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50197084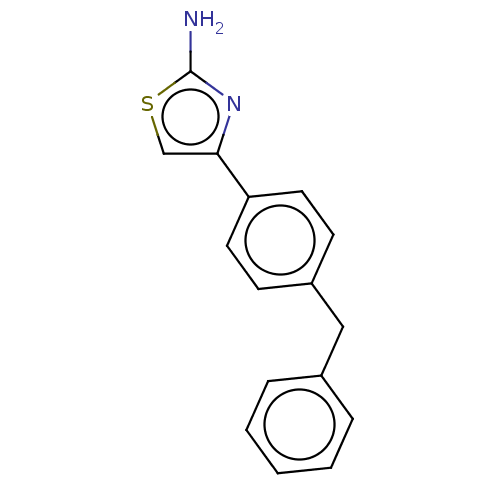 (CHEMBL3883608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Binding affinity to beta2 adrenergic receptor (unknown origin) by radioligand binding assay | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50530480 (CHEMBL4445524) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... | J Med Chem 62: 8443-8460 (2019) Article DOI: 10.1021/acs.jmedchem.9b00445 BindingDB Entry DOI: 10.7270/Q2V98CJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50530480 (CHEMBL4445524) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... | J Med Chem 62: 8443-8460 (2019) Article DOI: 10.1021/acs.jmedchem.9b00445 BindingDB Entry DOI: 10.7270/Q2V98CJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50264106 (CHEMBL3818875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561492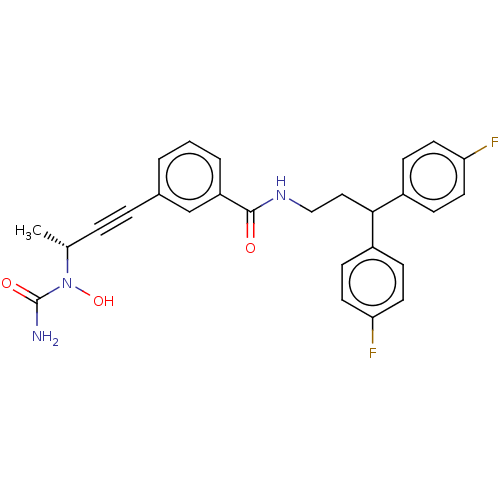 (CHEMBL4800490) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561490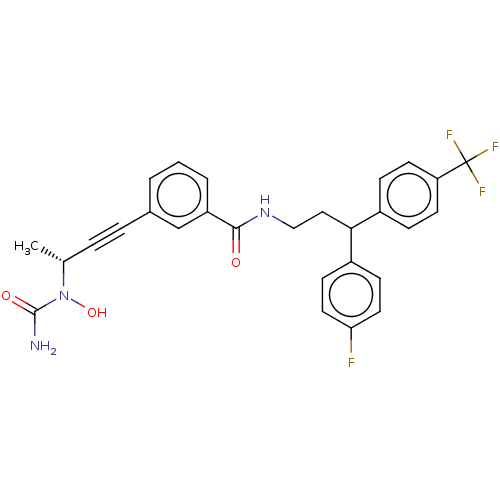 (CHEMBL4746942) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561483 (CHEMBL4795110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561486 (CHEMBL4759111) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561482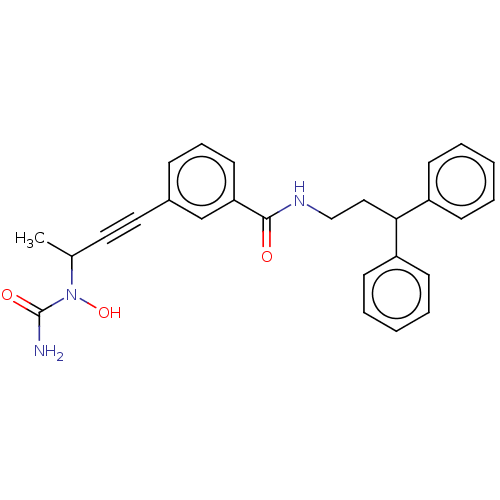 (CHEMBL4764099) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561489 (CHEMBL4745687) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561491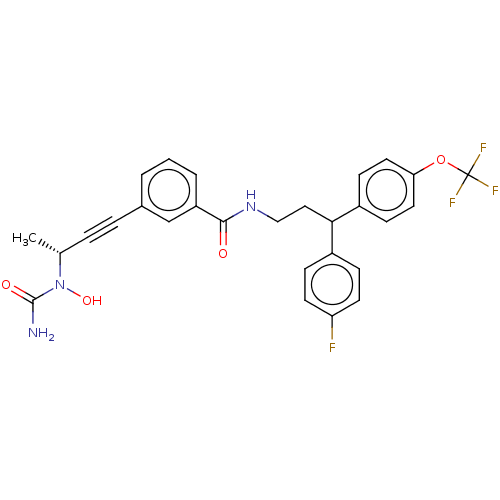 (CHEMBL4755533) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561488 (CHEMBL4745452) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561484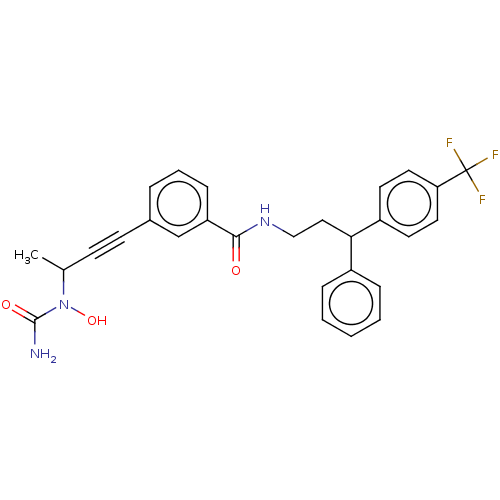 (CHEMBL4778283) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561495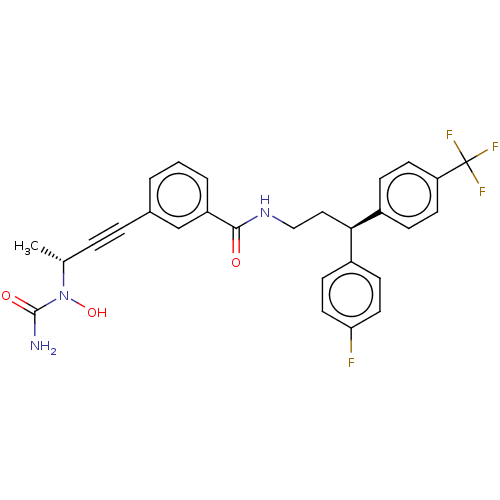 (CHEMBL4794423) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561494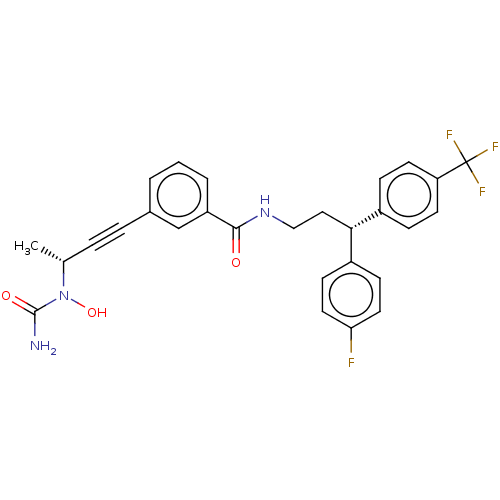 (CHEMBL4751593) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561487 (CHEMBL4759652) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561480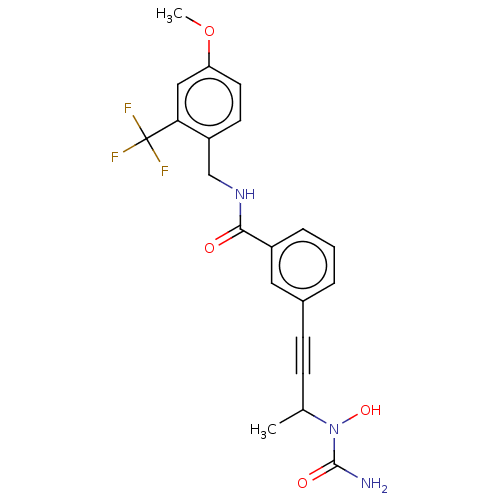 (CHEMBL4791222) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561474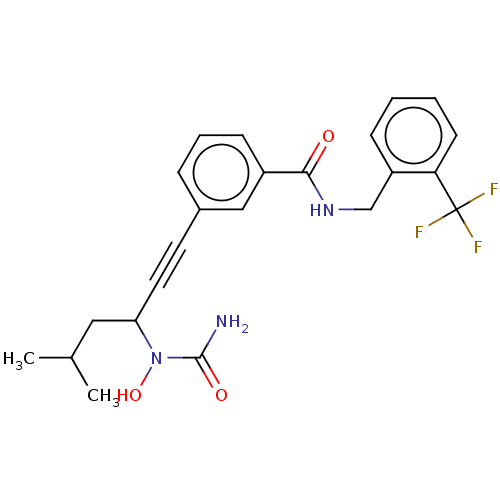 (CHEMBL4746544) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561493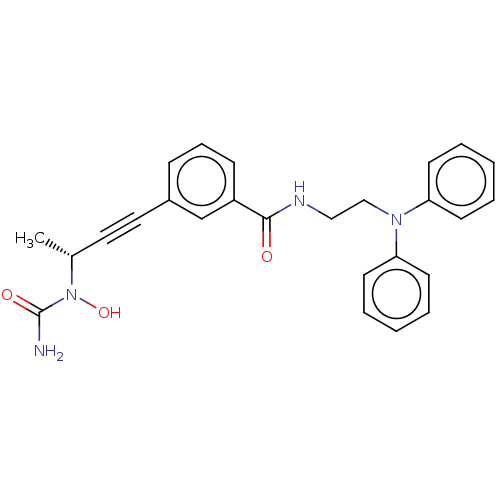 (CHEMBL4747688) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50116538 (3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561485 (CHEMBL4757630) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561473 (CHEMBL4753882) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561472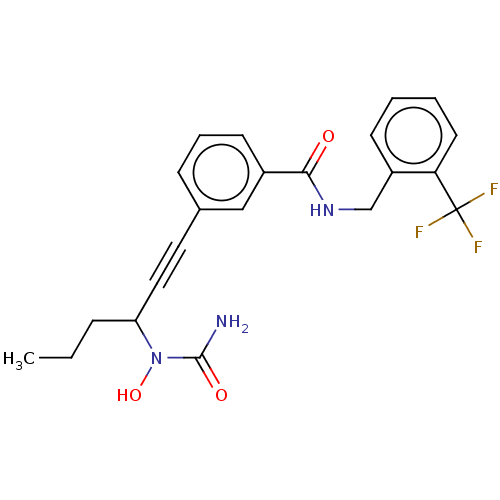 (CHEMBL4797528) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561478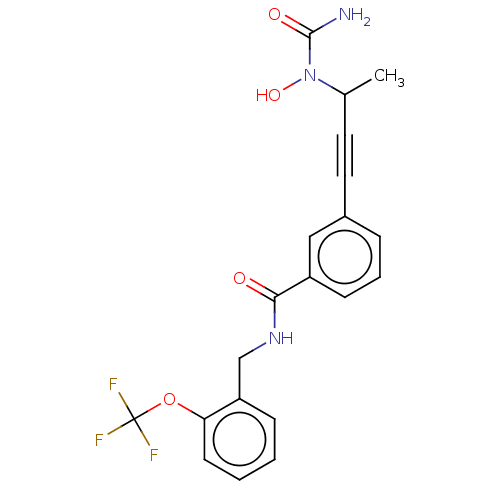 (CHEMBL4741953) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561471 (CHEMBL4781282) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50144900 (CHEMBL3764468) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... | J Med Chem 59: 61-81 (2016) Article DOI: 10.1021/acs.jmedchem.5b01239 BindingDB Entry DOI: 10.7270/Q2348N8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561481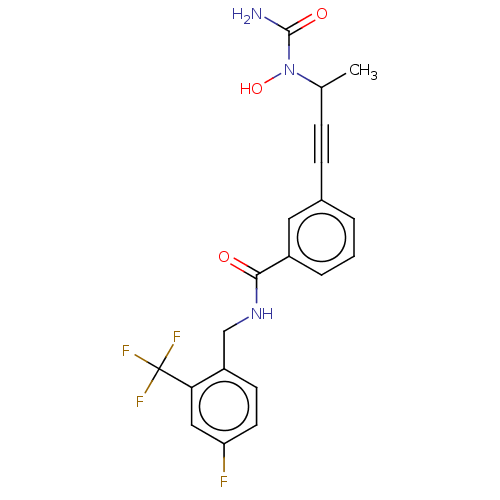 (CHEMBL4746074) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429375 (CHEMBL2336307) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50144830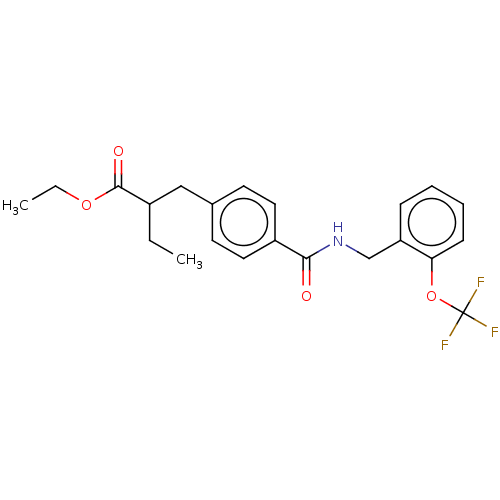 (CHEMBL3764004) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... | J Med Chem 59: 61-81 (2016) Article DOI: 10.1021/acs.jmedchem.5b01239 BindingDB Entry DOI: 10.7270/Q2348N8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50144891 (CHEMBL3764792) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... | J Med Chem 59: 61-81 (2016) Article DOI: 10.1021/acs.jmedchem.5b01239 BindingDB Entry DOI: 10.7270/Q2348N8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429376 (CHEMBL2336306) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429365 (CHEMBL2336297) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429364 (CHEMBL2336298) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561479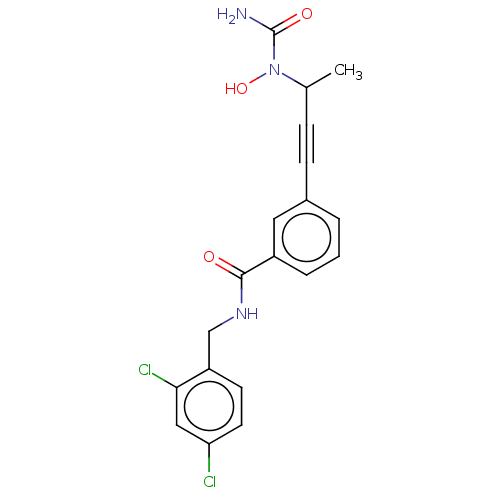 (CHEMBL4782144) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50144899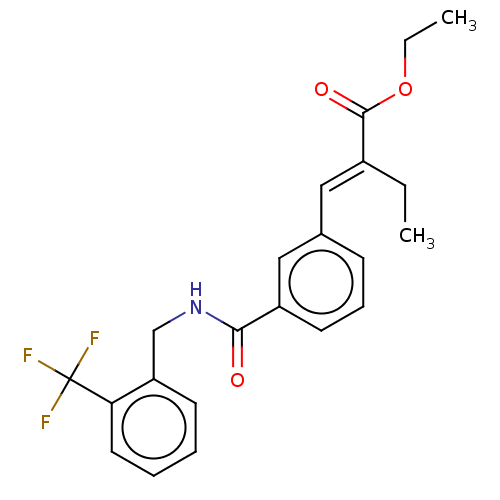 (CHEMBL3765080) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... | J Med Chem 59: 61-81 (2016) Article DOI: 10.1021/acs.jmedchem.5b01239 BindingDB Entry DOI: 10.7270/Q2348N8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429367 (CHEMBL2336315) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429378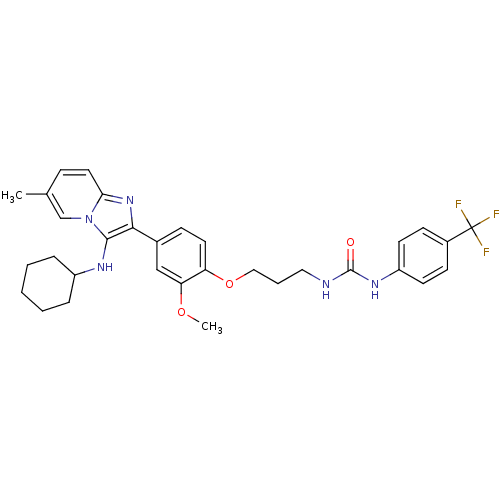 (CHEMBL2336304) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50144794 (CHEMBL3764773) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... | J Med Chem 59: 61-81 (2016) Article DOI: 10.1021/acs.jmedchem.5b01239 BindingDB Entry DOI: 10.7270/Q2348N8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561468 (CHEMBL4745567) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50429373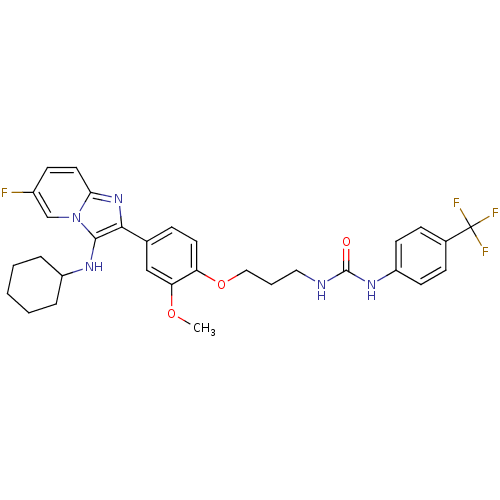 (CHEMBL2336309) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50365632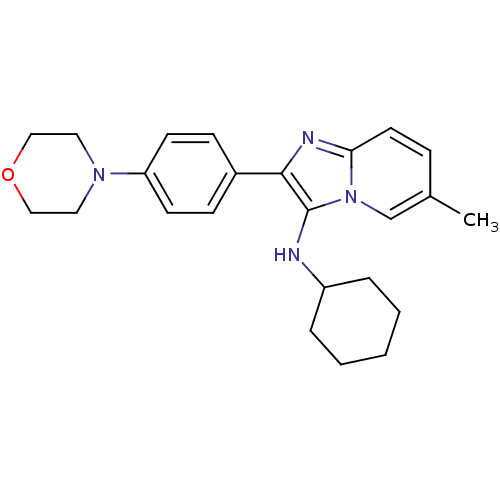 (CHEMBL1957971) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in Escherichia coli using 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methylester-2-o... | J Med Chem 56: 1777-81 (2013) Article DOI: 10.1021/jm301617j BindingDB Entry DOI: 10.7270/Q2377B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561469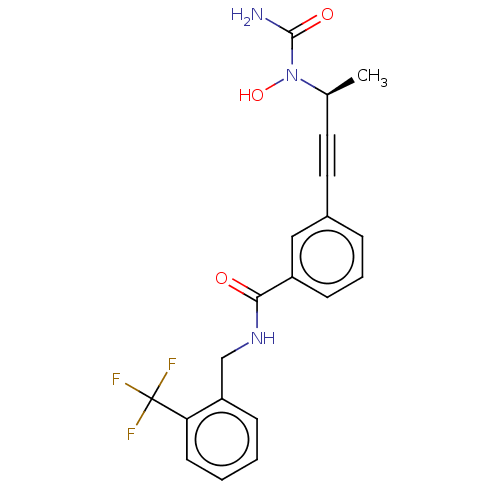 (CHEMBL4754077) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50197085 (CHEMBL3921982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561470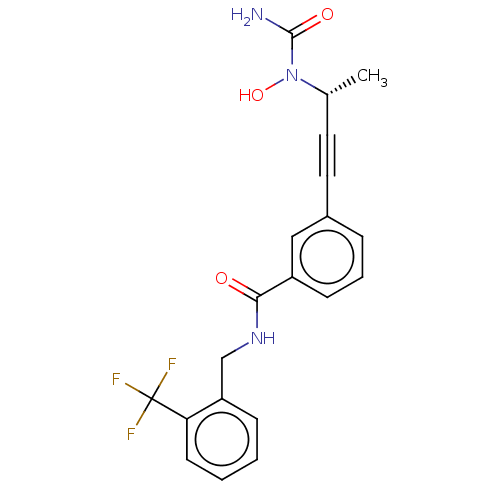 (CHEMBL4763763) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 323 total ) | Next | Last >> |