

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117522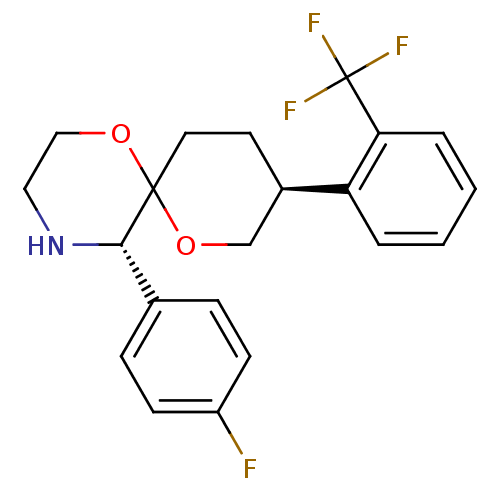 ((5S,6R,9S)-5-(4-Fluoro-phenyl)-9-(2-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to human Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117508 ((5S,6R,9R)-5-(4-Fluoro-phenyl)-9-(2-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to human Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403954 (CHEMBL2112366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50002660 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118664 (5-{3-[2-Methoxy-5-(5-trifluoromethyl-tetrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117523 (5-{(5S,10S)-5-(4-Fluoro-phenyl)-10-[2-methoxy-5-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117509 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-[2-methoxy-5-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118667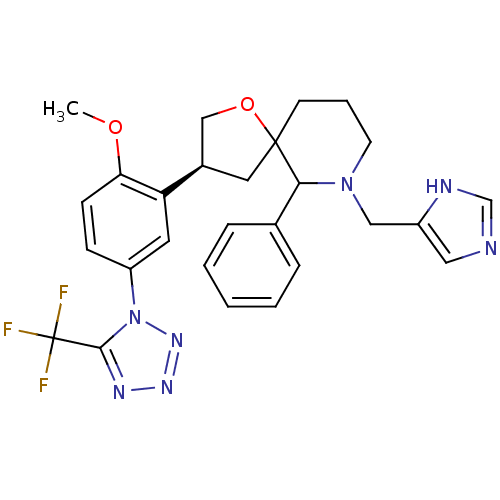 (7-(1H-Imidazol-4-ylmethyl)-3-[2-methoxy-5-(5-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118666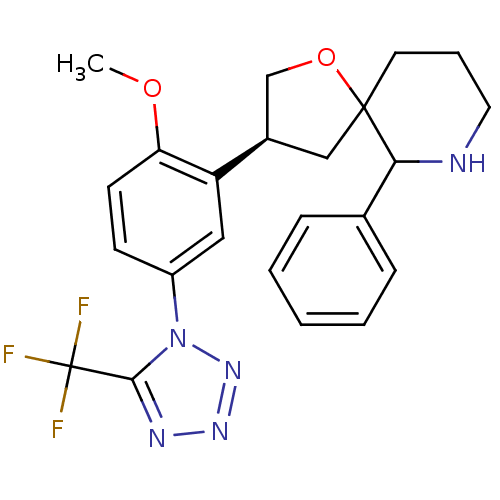 (3-[2-Methoxy-5-(5-trifluoromethyl-tetrazol-1-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029884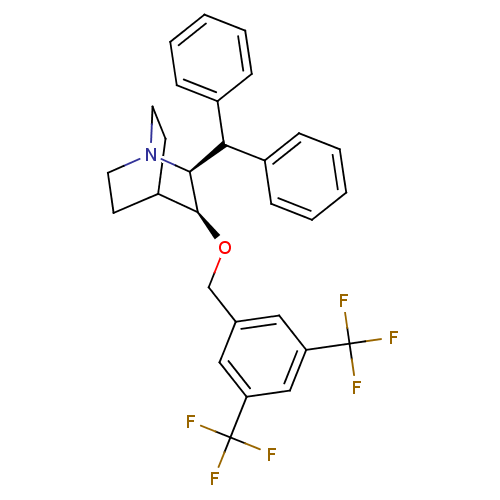 ((2S,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029878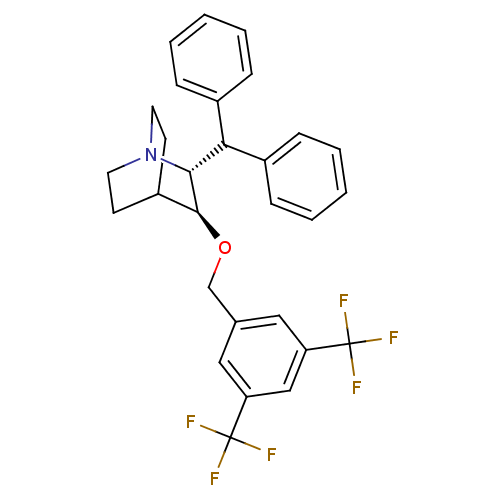 ((2R,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403953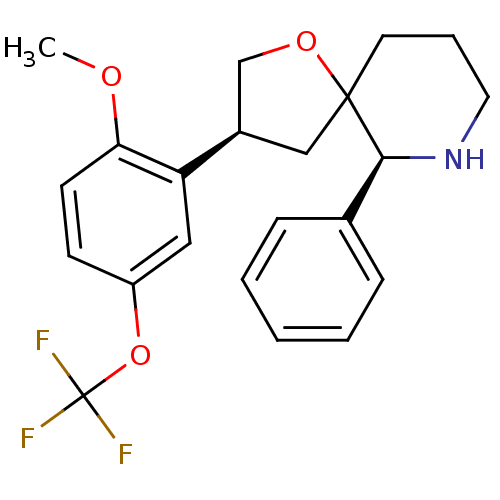 (CHEMBL2112368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029847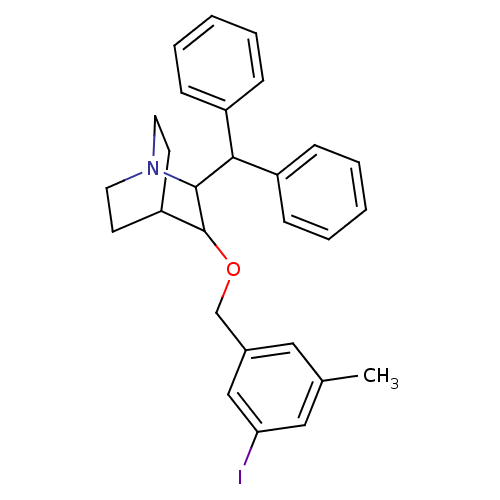 (2-Benzhydryl-3-(3-iodo-5-methyl-benzyloxy)-1-aza-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118671 (3-(2-Methoxy-5-trifluoromethoxy-phenyl)-6-phenyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125]Substance P Binding from human Neurokinin NK1 receptor in CHO Cells | Bioorg Med Chem Lett 3: 1361-1366 (1993) Article DOI: 10.1016/S0960-894X(00)80349-2 BindingDB Entry DOI: 10.7270/Q2251J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117515 (5-[(3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(2-methoxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118670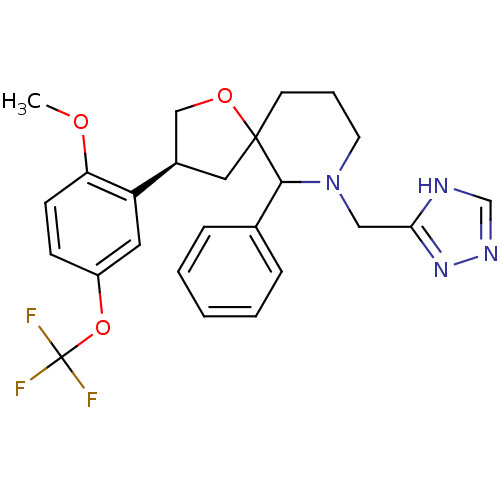 (3-(2-Methoxy-5-trifluoromethoxy-phenyl)-6-phenyl-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029871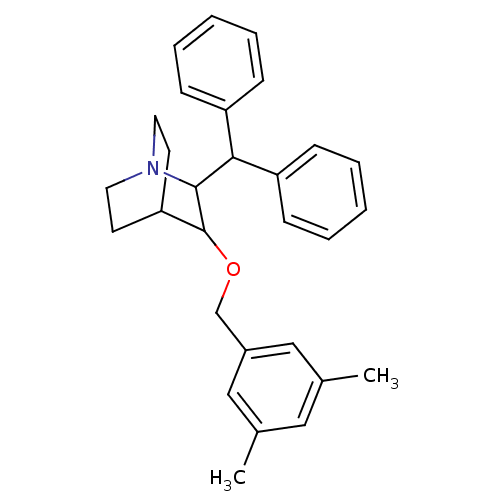 (2-Benzhydryl-3-(3,5-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029871 (2-Benzhydryl-3-(3,5-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029869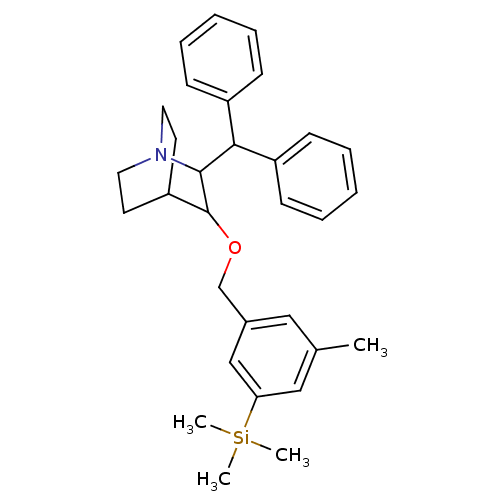 (2-Benzhydryl-3-(3-methyl-5-trimethylsilanyl-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281976 ((2S,3S)-2-Benzhydryl-3-(3,5-dimethyl-benzyloxy)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125]Substance P Binding from human Neurokinin NK1 receptor in CHO Cells | Bioorg Med Chem Lett 3: 1361-1366 (1993) Article DOI: 10.1016/S0960-894X(00)80349-2 BindingDB Entry DOI: 10.7270/Q2251J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117510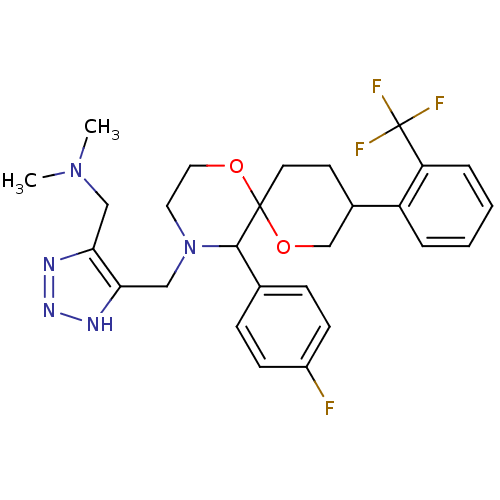 (CHEMBL84881 | {5-[5-(4-Fluoro-phenyl)-9-(2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117516 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(2-methoxy-5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117521 (CHEMBL85626 | {5-[5-(4-Fluoro-phenyl)-9-(2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029879 (2-Benzhydryl-3-(3-methoxy-5-methyl-benzyloxy)-1-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029879 (2-Benzhydryl-3-(3-methoxy-5-methyl-benzyloxy)-1-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029839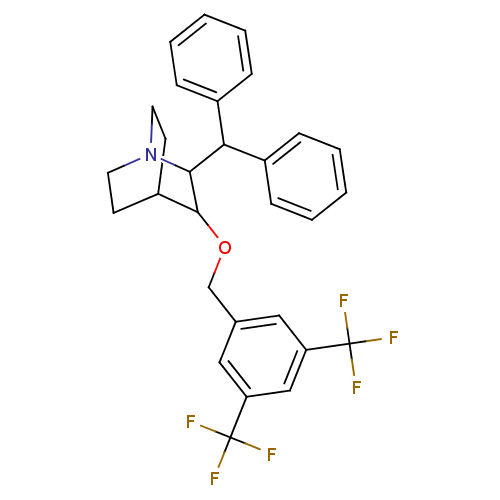 (2-Benzhydryl-3-(3,5-bis-trifluoromethyl-benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029839 (2-Benzhydryl-3-(3,5-bis-trifluoromethyl-benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029884 ((2S,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards wild type human Wild-type tachykinin receptor 1 expressed in Chinese hamster ovary cells | Bioorg Med Chem Lett 5: 1261-1264 (1995) Article DOI: 10.1016/0960-894X(95)00205-8 BindingDB Entry DOI: 10.7270/Q2RR1Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281976 ((2S,3S)-2-Benzhydryl-3-(3,5-dimethyl-benzyloxy)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards wild type human Wild-type tachykinin receptor 1 expressed in Chinese hamster ovary cells | Bioorg Med Chem Lett 5: 1261-1264 (1995) Article DOI: 10.1016/0960-894X(95)00205-8 BindingDB Entry DOI: 10.7270/Q2RR1Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117514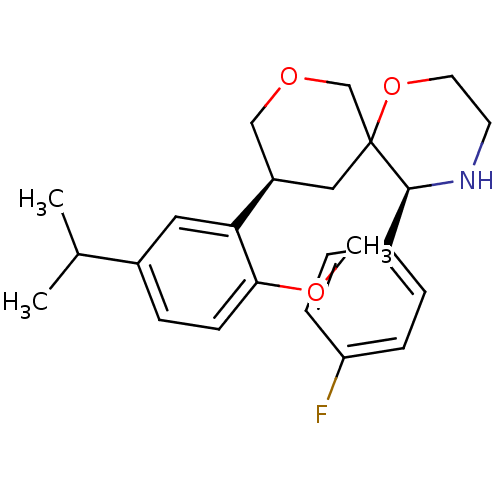 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(5-isopropyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029850 (3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-(1,2-diphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118662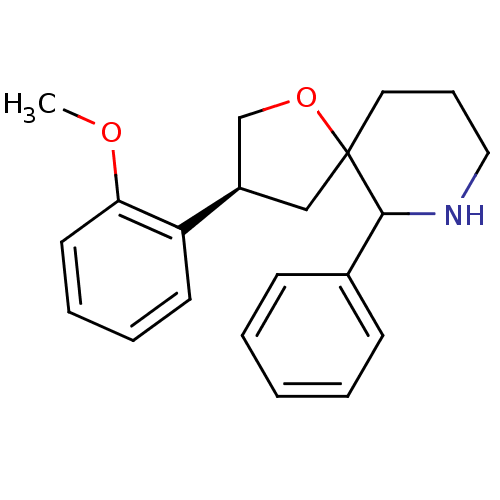 (3-(2-Methoxy-phenyl)-6-phenyl-1-oxa-7-aza-spiro[4....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029876 (3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-(cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029875 (2-Benzhydryl-3-(3,5-dimethoxy-benzyloxy)-1-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029875 (2-Benzhydryl-3-(3,5-dimethoxy-benzyloxy)-1-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029845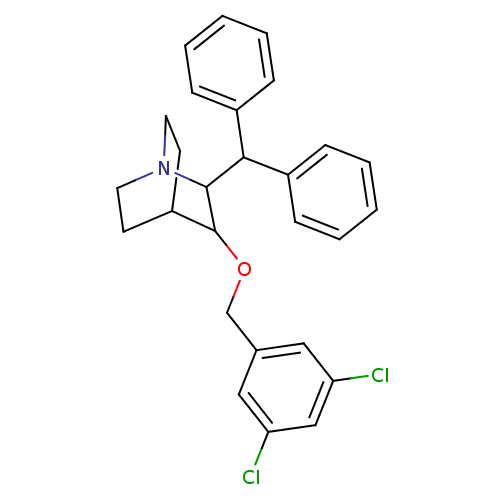 (2-Benzhydryl-3-(3,5-dichloro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029845 (2-Benzhydryl-3-(3,5-dichloro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117511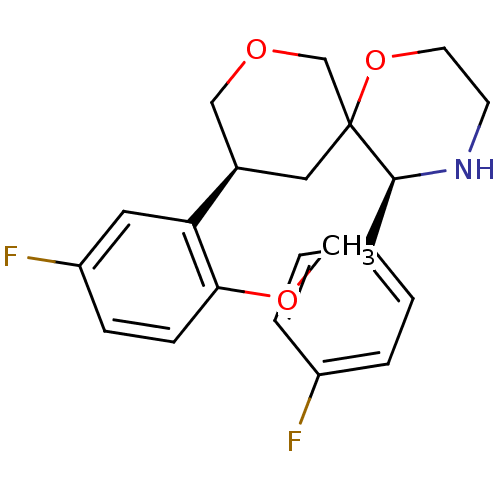 ((3S,5S,10S)-10-(5-Fluoro-2-methoxy-phenyl)-5-(4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117518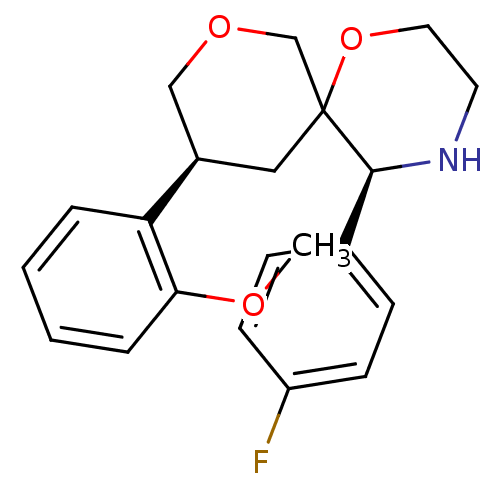 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(2-methoxy-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117524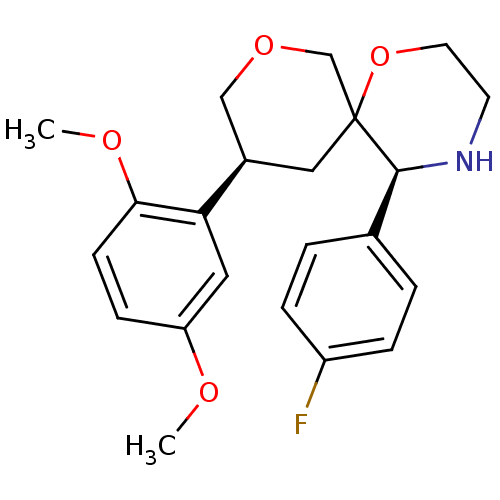 ((3S,5S,10S)-10-(2,5-Dimethoxy-phenyl)-5-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117506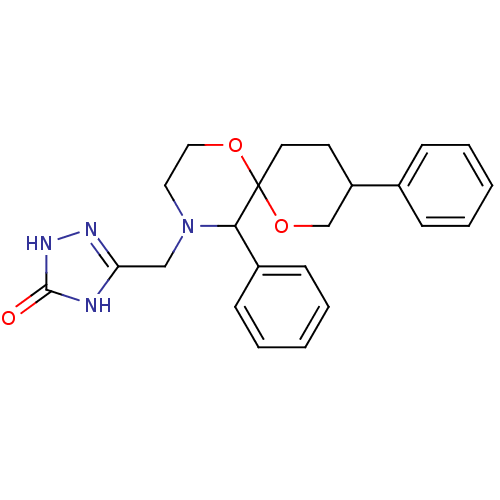 (5-(5,9-Diphenyl-1,7-dioxa-4-aza-spiro[5.5]undec-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029872 (2-Benzhydryl-3-(3-methyl-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281980 ((2S,3S)-2-Benzhydryl-3-(3-methyl-benzyloxy)-1-aza-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125]Substance P Binding from human Neurokinin NK1 receptor in CHO Cells | Bioorg Med Chem Lett 3: 1361-1366 (1993) Article DOI: 10.1016/S0960-894X(00)80349-2 BindingDB Entry DOI: 10.7270/Q2251J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117507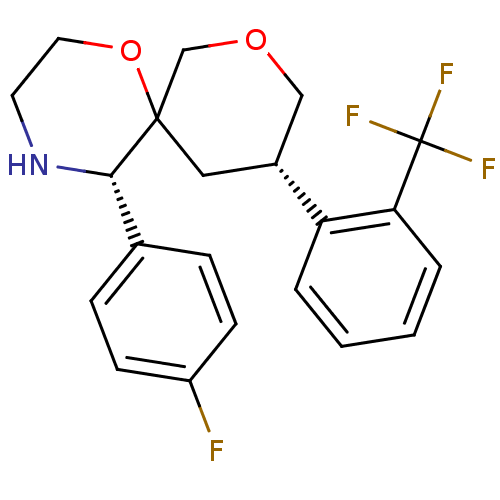 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(2-trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117513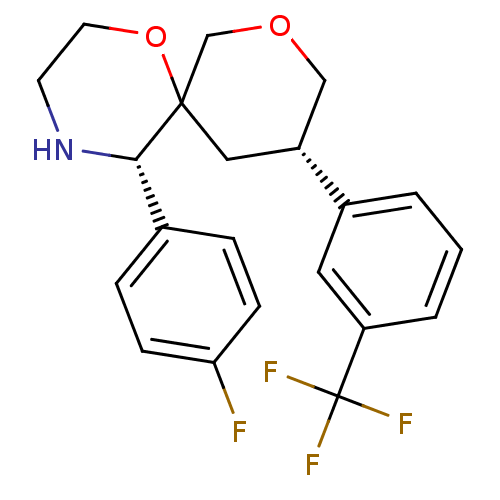 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(3-trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029842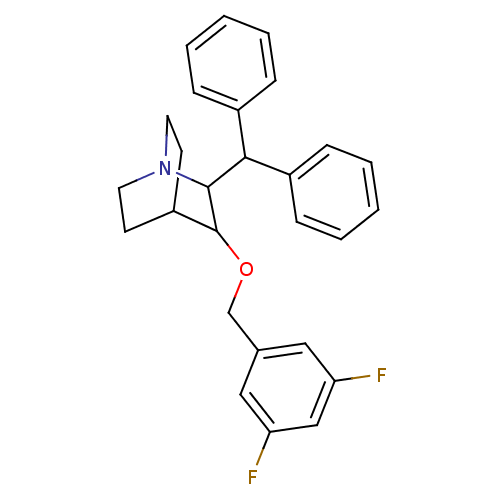 (2-Benzhydryl-3-(3,5-difluoro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029863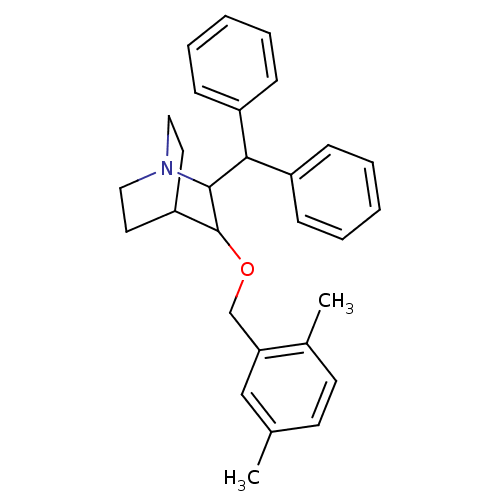 (2-Benzhydryl-3-(2,5-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029857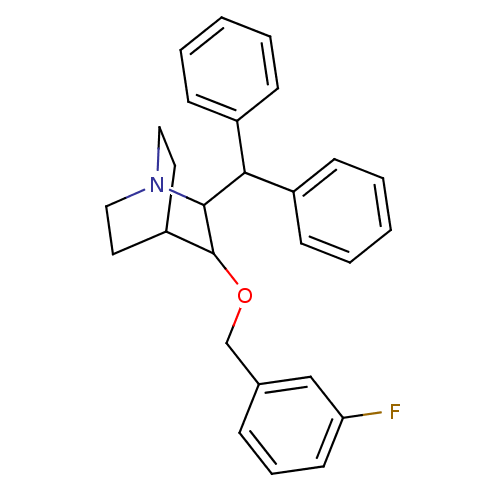 (2-Benzhydryl-3-(3-fluoro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 120 total ) | Next | Last >> |