

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473750 (US10844077, Compound I-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473766 (US10844077, Compound I-9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473764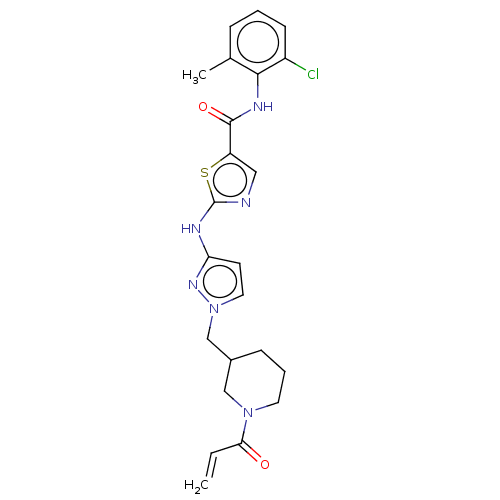 (US10844077, Compound II-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473751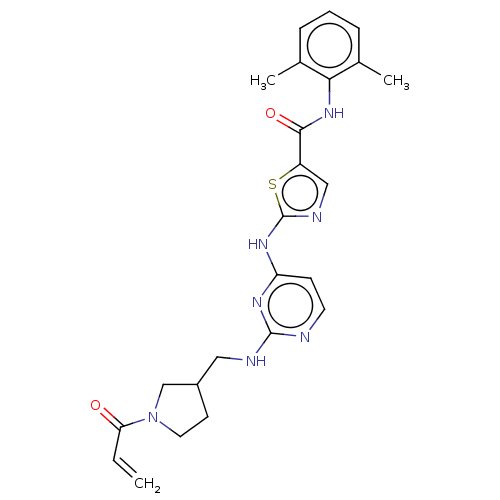 (US10844077, Compound I-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473751 (US10844077, Compound I-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473750 (US10844077, Compound I-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473746 (US10844077, Compound I-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473766 (US10844077, Compound I-9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473749 (US10844077, Compound I-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473701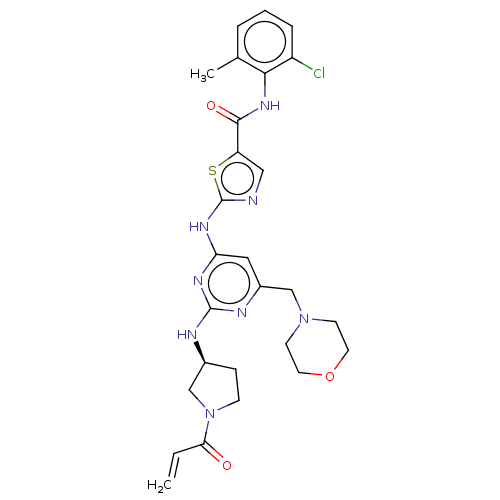 (US10844077, Compound I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473746 (US10844077, Compound I-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473748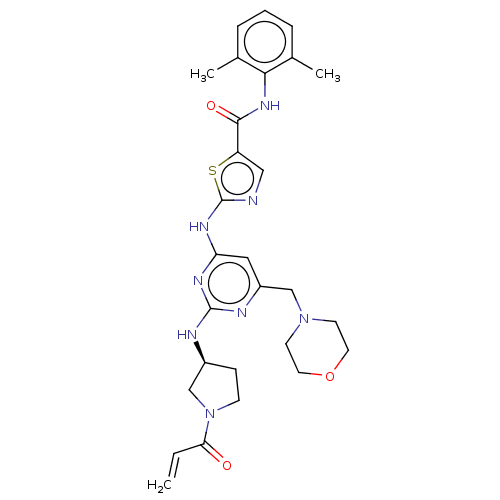 (US10844077, Compound I-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86632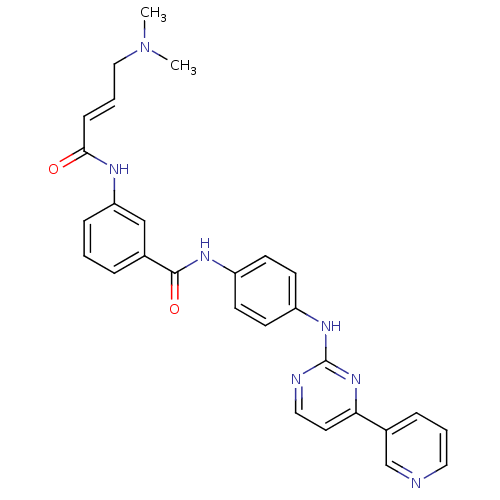 (JNK-IN-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length JNK3 (V28 to Q422 residues) expressed in mammalian expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473763 (US10844077, Compound II-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473749 (US10844077, Compound I-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86632 (JNK-IN-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human full length JNK1 (M1 to Q384 residues) expressed in mammalian expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473701 (US10844077, Compound I-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473748 (US10844077, Compound I-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473763 (US10844077, Compound II-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473752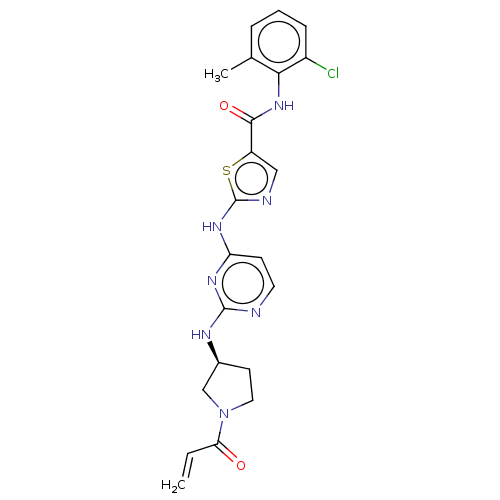 (US10844077, Compound I-10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473747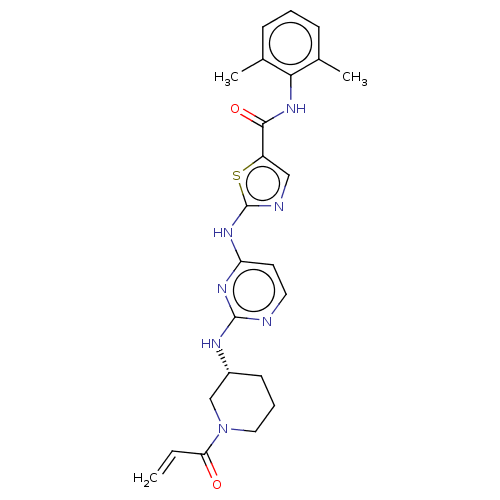 (US10844077, Compound I-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473764 (US10844077, Compound II-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86632 (JNK-IN-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human full length JNK2 (M1 to Q382 residues) expressed in mammalian expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473752 (US10844077, Compound I-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM163700 (N-(5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375312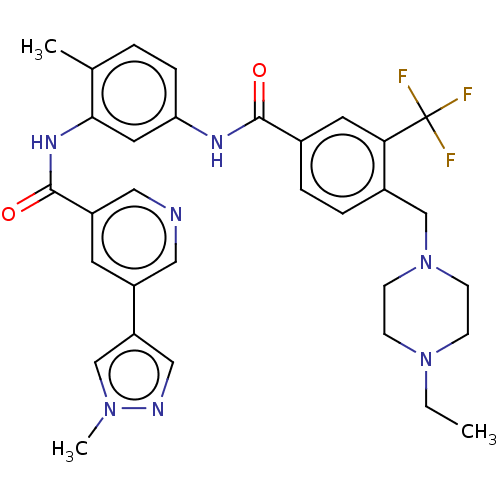 (US9908872, Compound (I-9)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473753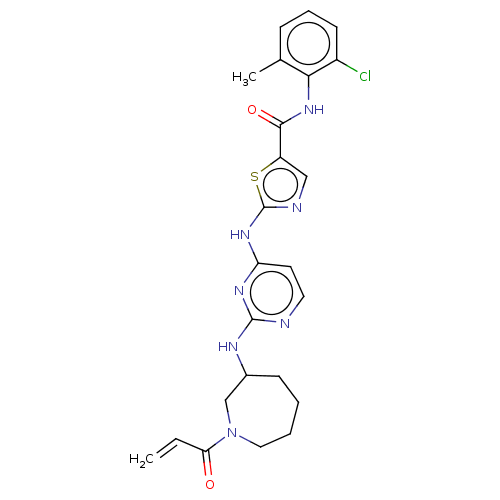 (US10844077, Compound I-11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473747 (US10844077, Compound I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM437992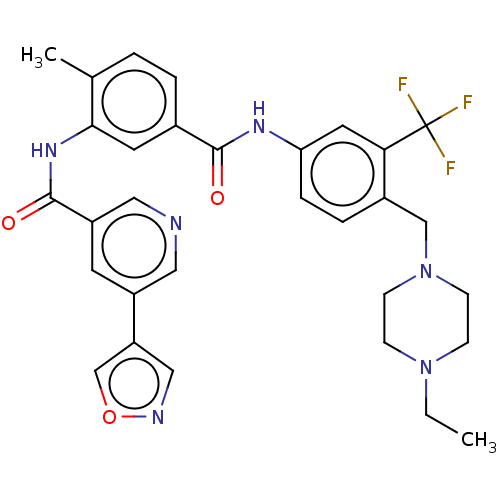 (US10597387, Compound (I-8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375310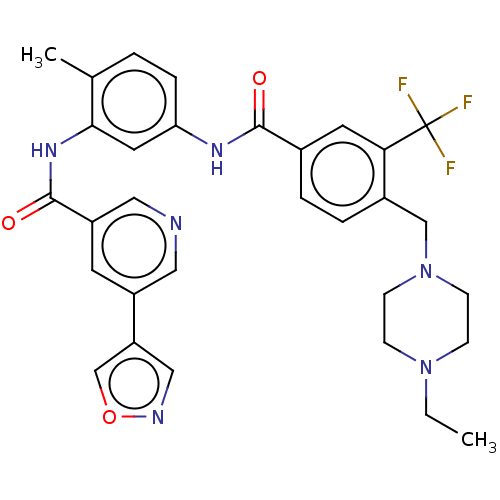 (US9908872, Compound (I-8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.33 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473753 (US10844077, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375276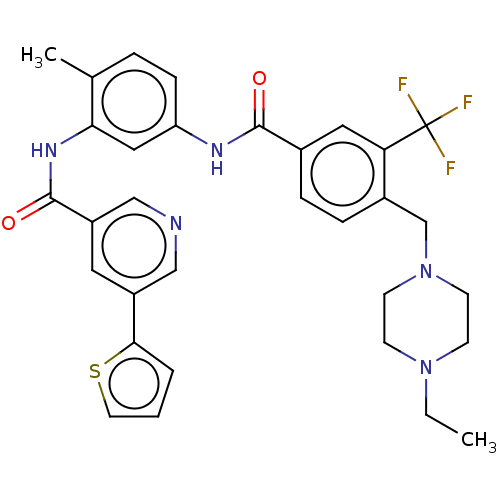 (US10597387, Compound (I-1) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM375276 (US10597387, Compound (I-1) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 1 (Homo sapiens (Human)) | BDBM50561821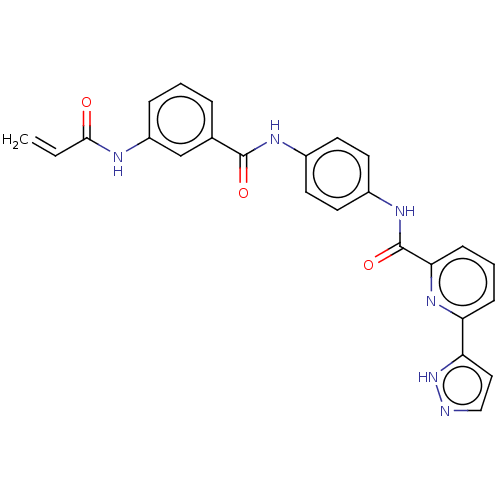 (CHEMBL4782804) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant IRAK1 (unknown origin) by Invitrogen adapta assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 1 (Homo sapiens (Human)) | BDBM50373415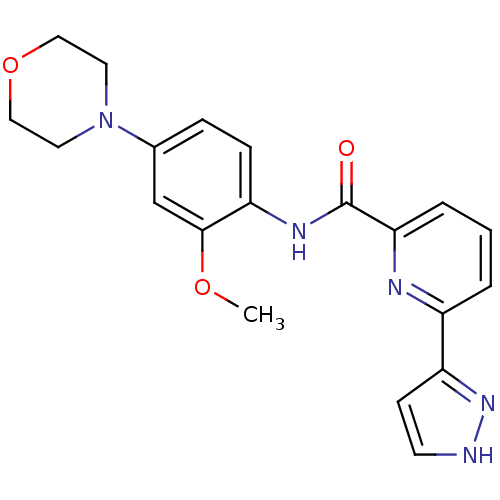 (CHEMBL256713) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length IRAK1 (R194 to S712 residues) expressed in mammalian expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473758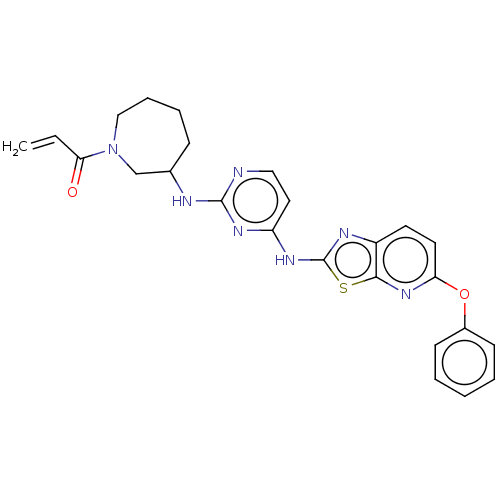 (US10844077, Compound III-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 1 (Homo sapiens (Human)) | BDBM86632 (JNK-IN-7) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length IRAK1 (R194 to S712 residues) expressed in mammalian expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM439303 (US10633348, Compound (A-17)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50373415 (CHEMBL256713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length IRAK4 (M1 to S460 residues) expressed in mammalian expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM439316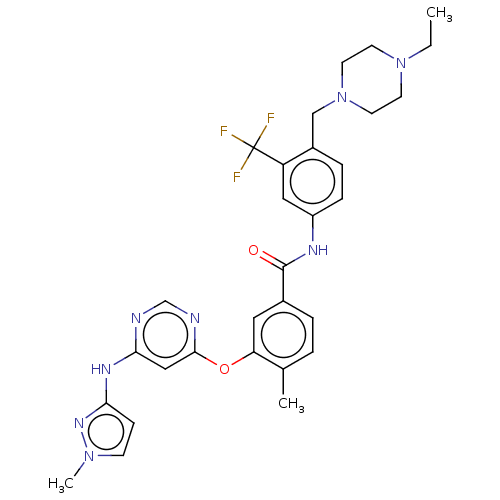 (US10633348, Compound (A-12)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473756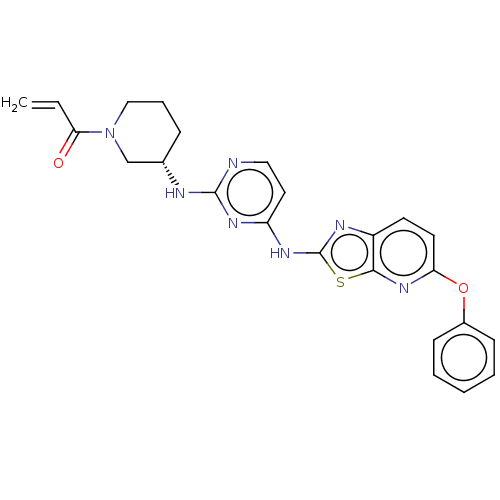 (US10844077, Compound III-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM473765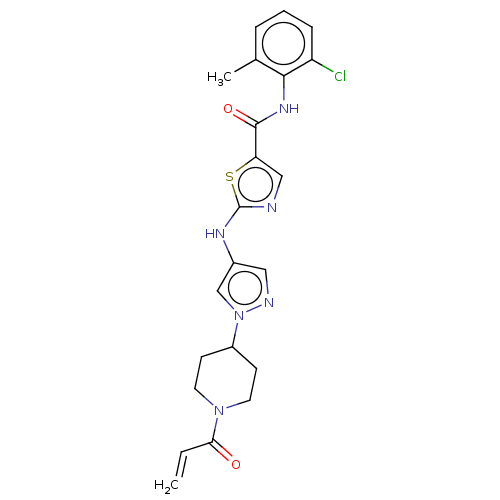 (US10844077, Compound II-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM375281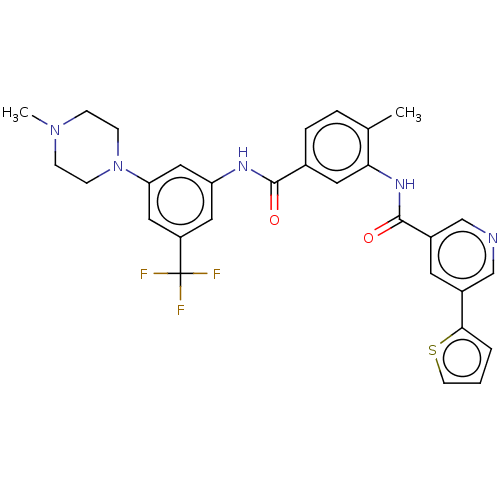 (US10597387, Compound (I-4) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375281 (US10597387, Compound (I-4) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM375276 (US10597387, Compound (I-1) | US9908872, Compound (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM375276 (US10597387, Compound (I-1) | US9908872, Compound (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 1 (Homo sapiens (Human)) | BDBM50561824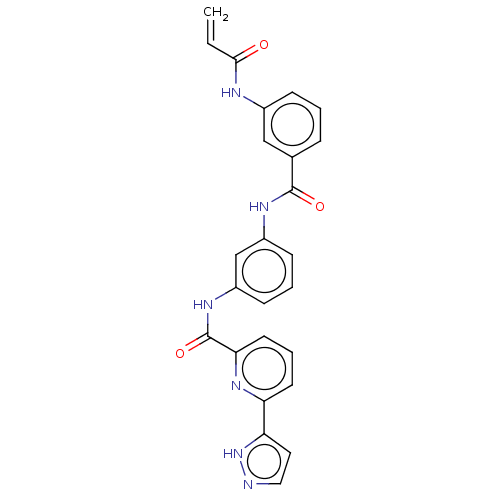 (CHEMBL4784448) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant IRAK1 (unknown origin) by Invitrogen adapta assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00378 BindingDB Entry DOI: 10.7270/Q2N01B75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM163700 (N-(5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM375312 (US9908872, Compound (I-9)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473765 (US10844077, Compound II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |