

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50503718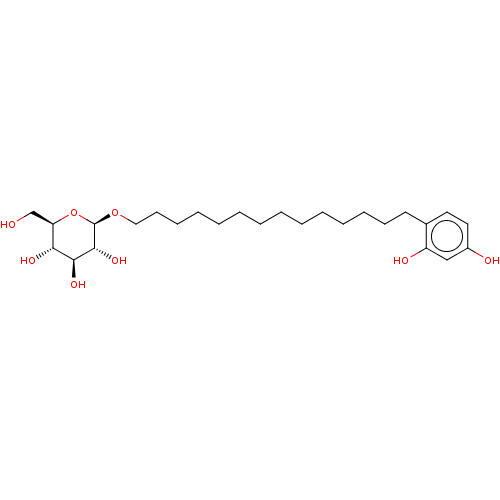 (CHEMBL4439507) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50503713 (CHEMBL4462064) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50292636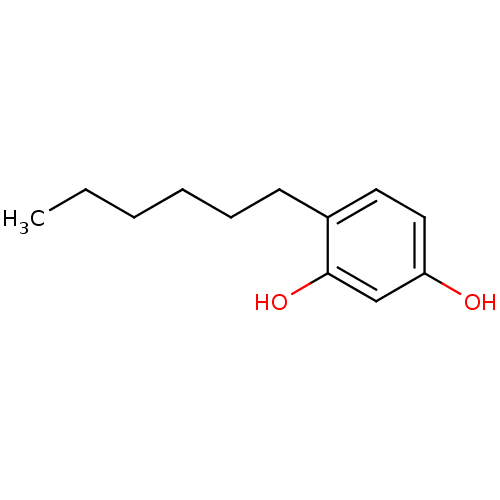 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50503714 (CHEMBL4456030) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50503717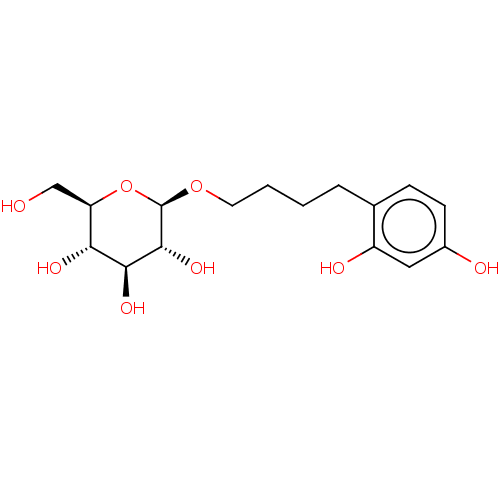 (CHEMBL4465094) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50120802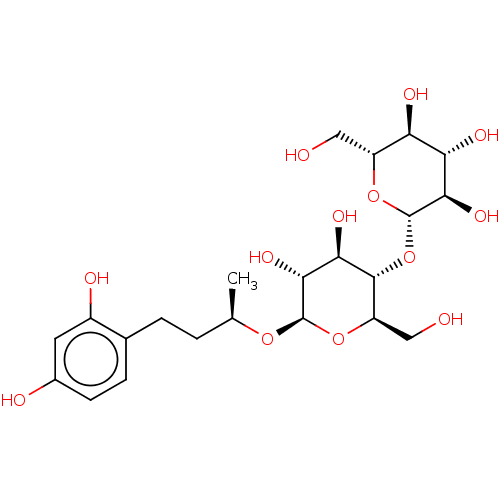 (CHEMBL3100130) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120802 (CHEMBL3100130) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120803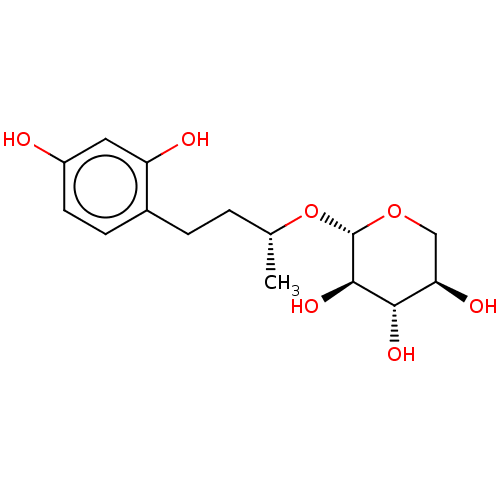 (CHEMBL3100134) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50120803 (CHEMBL3100134) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120804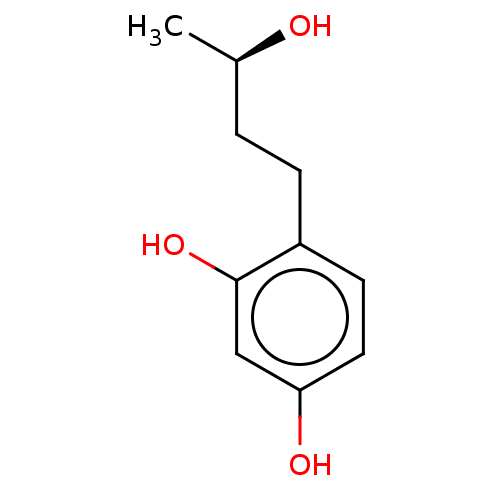 (CHEMBL3618459) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120800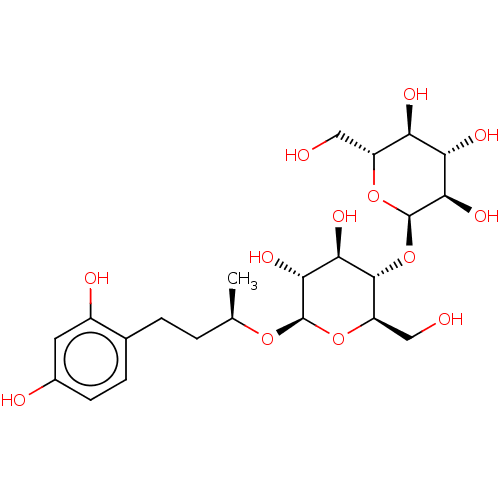 (CHEMBL3618460) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120813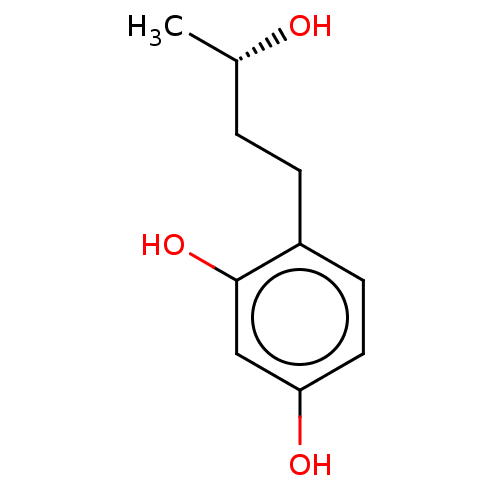 (CHEMBL3618457) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120806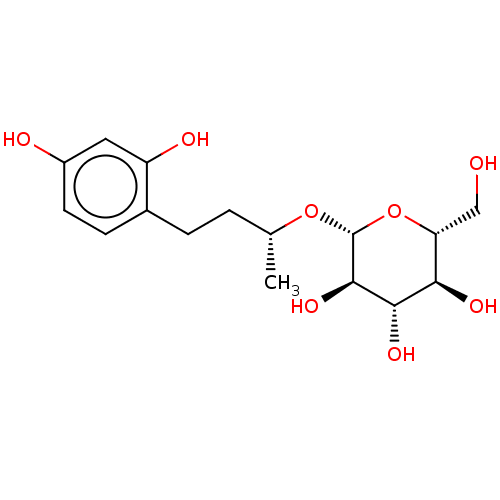 (CHEMBL3100132) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50120806 (CHEMBL3100132) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50503712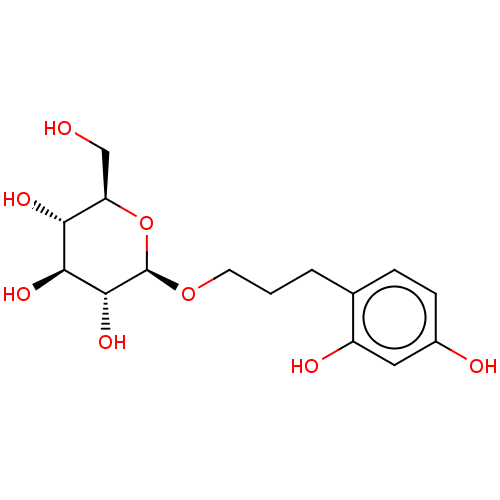 (CHEMBL4541503) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120810 (CHEMBL3100135) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50120810 (CHEMBL3100135) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120807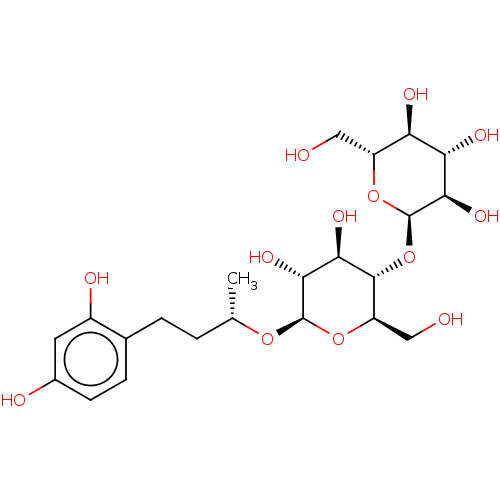 (CHEMBL3618458) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120811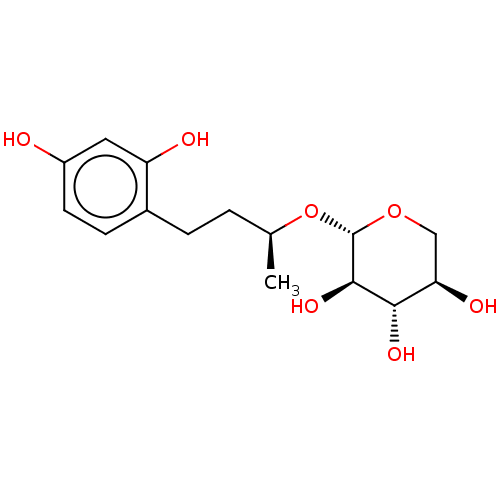 (CHEMBL3100133) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50120811 (CHEMBL3100133) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50120814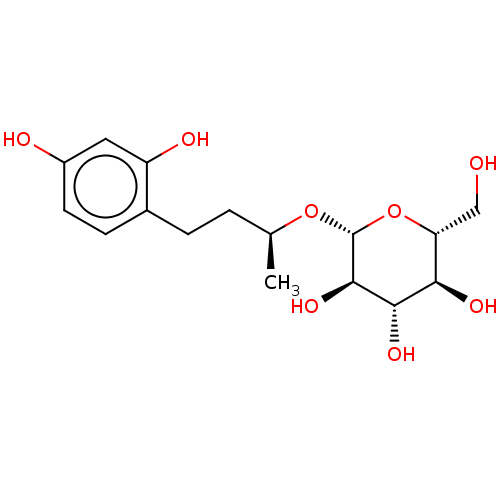 (CHEMBL3100131) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50120814 (CHEMBL3100131) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) Article DOI: 10.1016/j.bmc.2015.09.014 BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50503716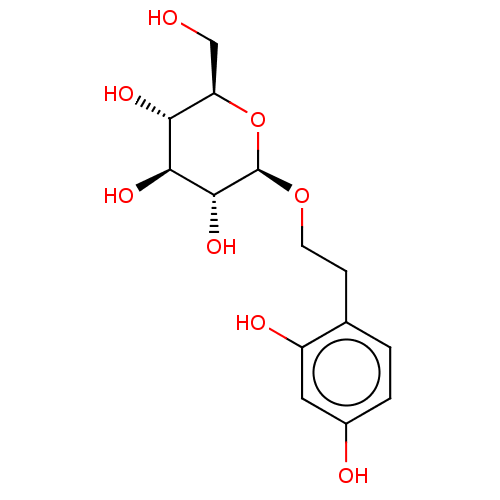 (CHEMBL4453823) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50503715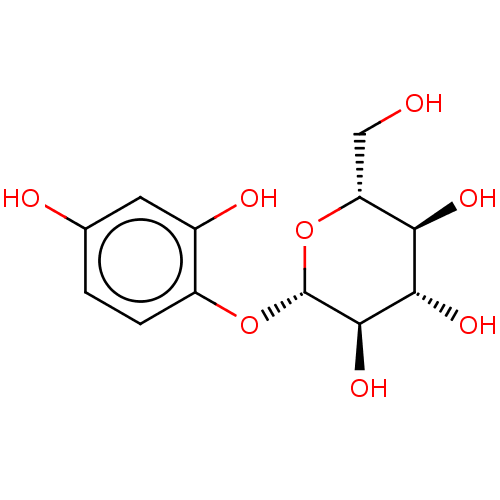 (CHEMBL4586957) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate preincubated with substrate for 5 mins followed by enzyme addition and measured i... | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 BindingDB Entry DOI: 10.7270/Q22V2KCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||