 Found 121 hits with Last Name = 'nagahara' and Initial = 't'
Found 121 hits with Last Name = 'nagahara' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50121214
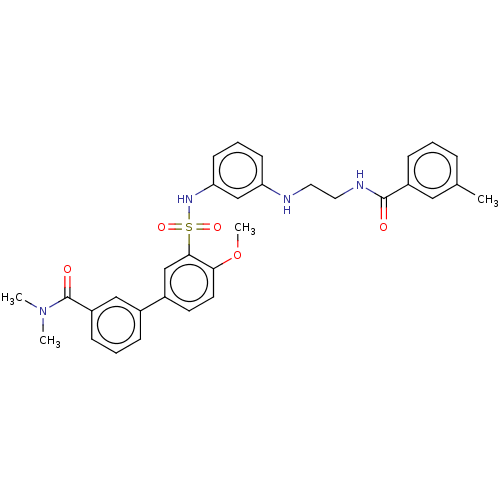
(CHEMBL3623075)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1cccc(NCCNC(=O)c2cccc(C)c2)c1)-c1cccc(c1)C(=O)N(C)C Show InChI InChI=1S/C32H34N4O5S/c1-22-8-5-10-25(18-22)31(37)34-17-16-33-27-12-7-13-28(21-27)35-42(39,40)30-20-24(14-15-29(30)41-4)23-9-6-11-26(19-23)32(38)36(2)3/h5-15,18-21,33,35H,16-17H2,1-4H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [125I]orexin-A from human OX1R expressed in CHO cell membranes by scintillation counting method |
J Med Chem 58: 7931-7 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00988
BindingDB Entry DOI: 10.7270/Q2GH9KSW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50121214

(CHEMBL3623075)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1cccc(NCCNC(=O)c2cccc(C)c2)c1)-c1cccc(c1)C(=O)N(C)C Show InChI InChI=1S/C32H34N4O5S/c1-22-8-5-10-25(18-22)31(37)34-17-16-33-27-12-7-13-28(21-27)35-42(39,40)30-20-24(14-15-29(30)41-4)23-9-6-11-26(19-23)32(38)36(2)3/h5-15,18-21,33,35H,16-17H2,1-4H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [125I]orexin-A from human OX2R expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 58: 7931-7 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00988
BindingDB Entry DOI: 10.7270/Q2GH9KSW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146539
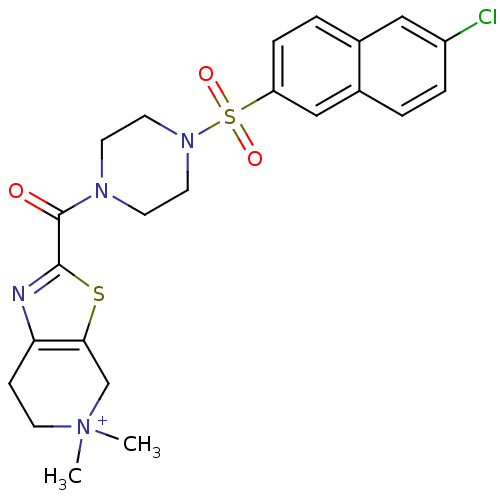
(2-[4-(6-Chloro-naphthalene-2-sulfonyl)-piperazine-...)Show SMILES C[N+]1(C)CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H26ClN4O3S2/c1-28(2)12-7-20-21(15-28)32-22(25-20)23(29)26-8-10-27(11-9-26)33(30,31)19-6-4-16-13-18(24)5-3-17(16)14-19/h3-6,13-14H,7-12,15H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146538
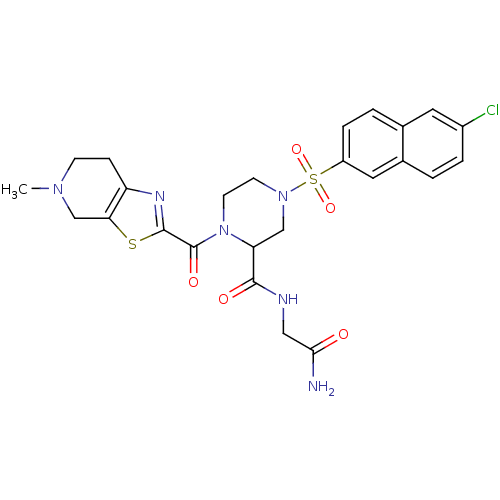
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCC(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H27ClN6O5S2/c1-30-7-6-19-21(14-30)38-24(29-19)25(35)32-9-8-31(13-20(32)23(34)28-12-22(27)33)39(36,37)18-5-3-15-10-17(26)4-2-16(15)11-18/h2-5,10-11,20H,6-9,12-14H2,1H3,(H2,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146536
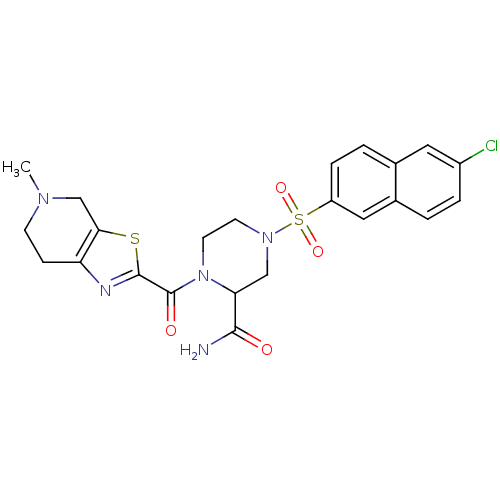
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN5O4S2/c1-27-7-6-18-20(13-27)34-22(26-18)23(31)29-9-8-28(12-19(29)21(25)30)35(32,33)17-5-3-14-10-16(24)4-2-15(14)11-17/h2-5,10-11,19H,6-9,12-13H2,1H3,(H2,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146536

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN5O4S2/c1-27-7-6-18-20(13-27)34-22(26-18)23(31)29-9-8-28(12-19(29)21(25)30)35(32,33)17-5-3-14-10-16(24)4-2-15(14)11-17/h2-5,10-11,19H,6-9,12-13H2,1H3,(H2,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146532
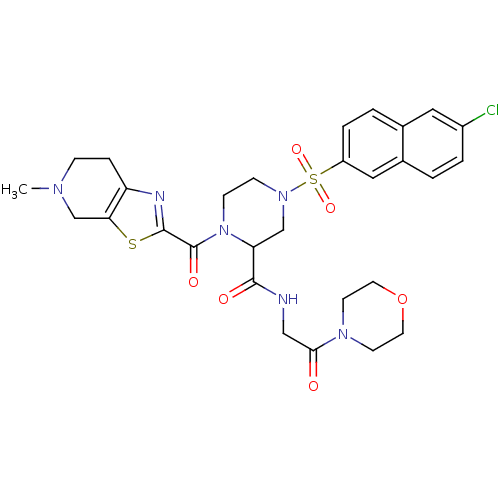
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCC(=O)N1CCOCC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C29H33ClN6O6S2/c1-33-7-6-23-25(18-33)43-28(32-23)29(39)36-9-8-35(17-24(36)27(38)31-16-26(37)34-10-12-42-13-11-34)44(40,41)22-5-3-19-14-21(30)4-2-20(19)15-22/h2-5,14-15,24H,6-13,16-18H2,1H3,(H,31,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146531
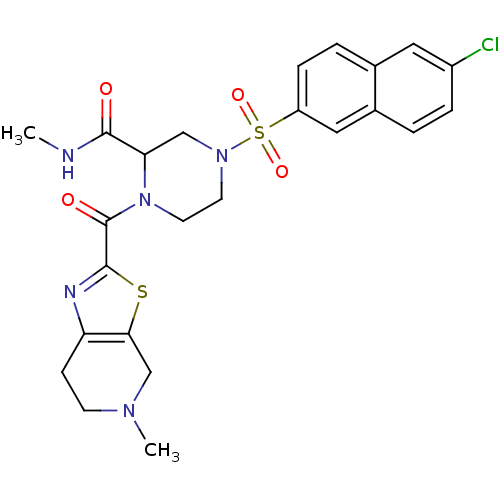
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CNC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C24H26ClN5O4S2/c1-26-22(31)20-13-29(36(33,34)18-6-4-15-11-17(25)5-3-16(15)12-18)9-10-30(20)24(32)23-27-19-7-8-28(2)14-21(19)35-23/h3-6,11-12,20H,7-10,13-14H2,1-2H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433895
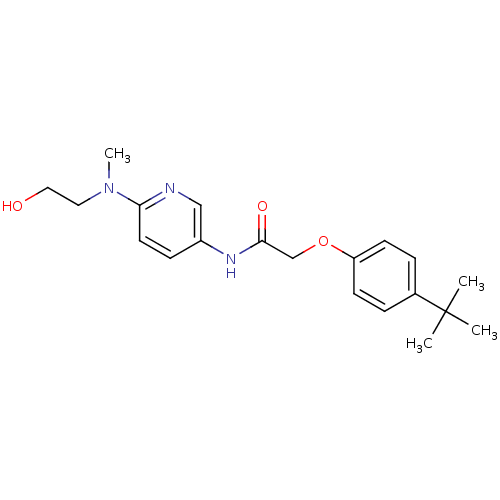
(CHEMBL2380435)Show SMILES CN(CCO)c1ccc(NC(=O)COc2ccc(cc2)C(C)(C)C)cn1 Show InChI InChI=1S/C20H27N3O3/c1-20(2,3)15-5-8-17(9-6-15)26-14-19(25)22-16-7-10-18(21-13-16)23(4)11-12-24/h5-10,13,24H,11-12,14H2,1-4H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433893
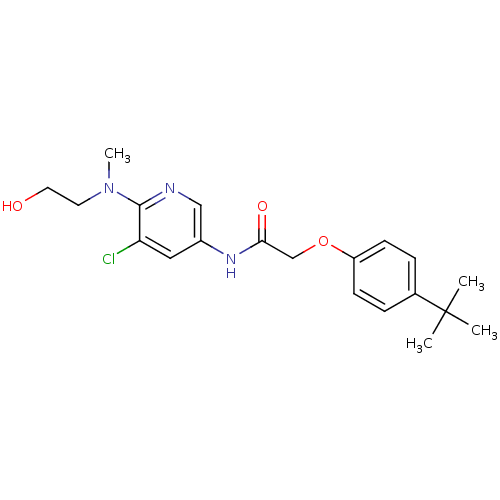
(CHEMBL2380437)Show SMILES CN(CCO)c1ncc(NC(=O)COc2ccc(cc2)C(C)(C)C)cc1Cl Show InChI InChI=1S/C20H26ClN3O3/c1-20(2,3)14-5-7-16(8-6-14)27-13-18(26)23-15-11-17(21)19(22-12-15)24(4)9-10-25/h5-8,11-12,25H,9-10,13H2,1-4H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146537

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CC(C)NC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C26H30ClN5O4S2/c1-16(2)28-24(33)22-14-31(38(35,36)20-7-5-17-12-19(27)6-4-18(17)13-20)10-11-32(22)26(34)25-29-21-8-9-30(3)15-23(21)37-25/h4-7,12-13,16,22H,8-11,14-15H2,1-3H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433897
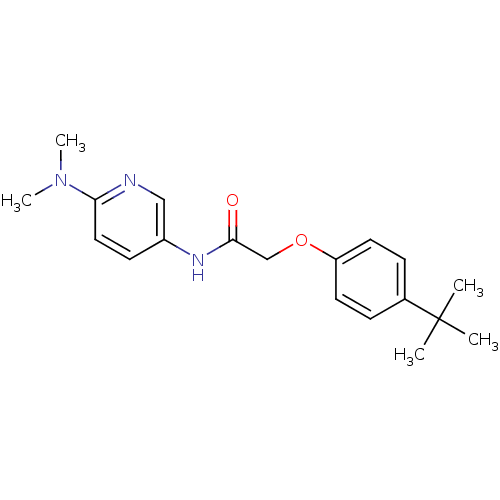
(CHEMBL2380433)Show InChI InChI=1S/C19H25N3O2/c1-19(2,3)14-6-9-16(10-7-14)24-13-18(23)21-15-8-11-17(20-12-15)22(4)5/h6-12H,13H2,1-5H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146535
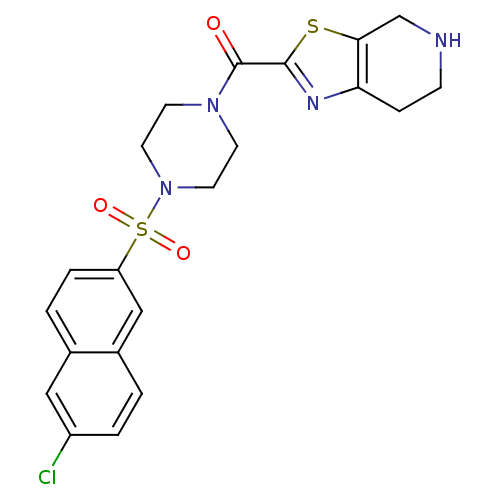
(CHEMBL99483 | [4-(6-Chloro-naphthalene-2-sulfonyl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1nc2CCNCc2s1 Show InChI InChI=1S/C21H21ClN4O3S2/c22-16-3-1-15-12-17(4-2-14(15)11-16)31(28,29)26-9-7-25(8-10-26)21(27)20-24-18-5-6-23-13-19(18)30-20/h1-4,11-12,23H,5-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146535

(CHEMBL99483 | [4-(6-Chloro-naphthalene-2-sulfonyl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1nc2CCNCc2s1 Show InChI InChI=1S/C21H21ClN4O3S2/c22-16-3-1-15-12-17(4-2-14(15)11-16)31(28,29)26-9-7-25(8-10-26)21(27)20-24-18-5-6-23-13-19(18)30-20/h1-4,11-12,23H,5-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154263
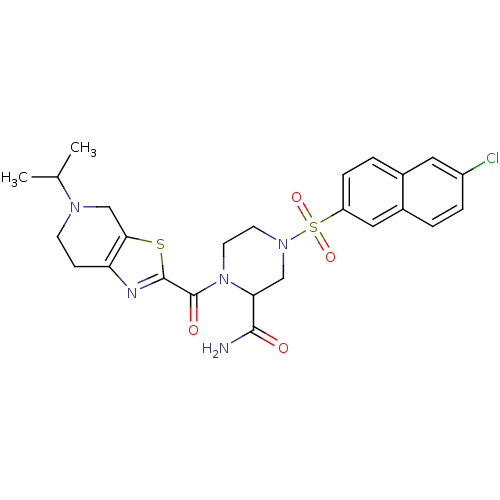
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-isopropyl...)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H28ClN5O4S2/c1-15(2)29-8-7-20-22(14-29)36-24(28-20)25(33)31-10-9-30(13-21(31)23(27)32)37(34,35)19-6-4-16-11-18(26)5-3-17(16)12-19/h3-6,11-12,15,21H,7-10,13-14H2,1-2H3,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154267

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(4,5,6,7-tet...)Show SMILES NC(=O)C1CN(CCN1C(=O)c1nc2CCNCc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H22ClN5O4S2/c23-15-3-1-14-10-16(4-2-13(14)9-15)34(31,32)27-7-8-28(18(12-27)20(24)29)22(30)21-26-17-5-6-25-11-19(17)33-21/h1-4,9-10,18,25H,5-8,11-12H2,(H2,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433885
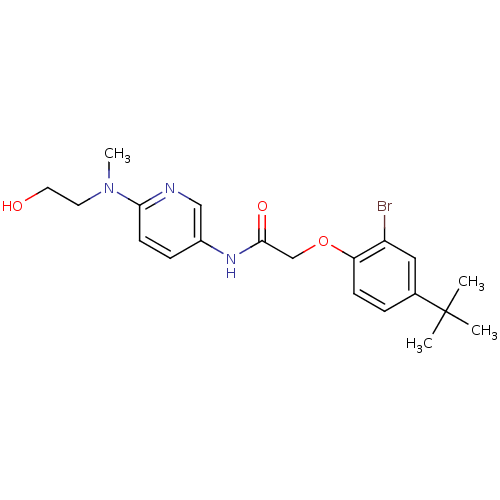
(CHEMBL2380571)Show SMILES CN(CCO)c1ccc(NC(=O)COc2ccc(cc2Br)C(C)(C)C)cn1 Show InChI InChI=1S/C20H26BrN3O3/c1-20(2,3)14-5-7-17(16(21)11-14)27-13-19(26)23-15-6-8-18(22-12-15)24(4)9-10-25/h5-8,11-12,25H,9-10,13H2,1-4H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041220
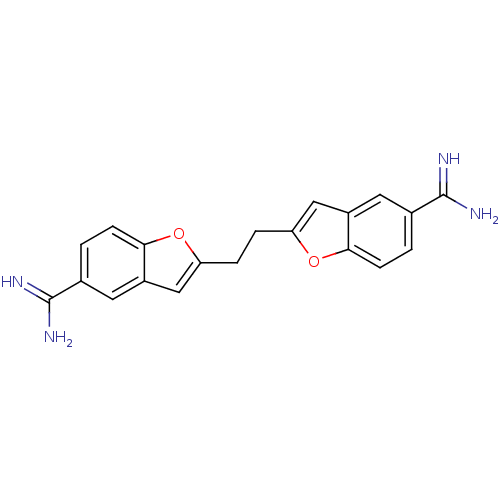
(1,2-bis(5-amidinobenzofuran-2-yl)ethane | 2-(2-{5-...)Show SMILES NC(=N)c1ccc2oc(CCc3cc4cc(ccc4o3)C(N)=N)cc2c1 Show InChI InChI=1S/C20H18N4O2/c21-19(22)11-1-5-17-13(7-11)9-15(25-17)3-4-16-10-14-8-12(20(23)24)2-6-18(14)26-16/h1-2,5-10H,3-4H2,(H3,21,22)(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154264

(CHEMBL365654 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2cc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN3O3S2/c1-25-7-6-18-14-21(31-22(18)15-25)23(28)26-8-10-27(11-9-26)32(29,30)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433886
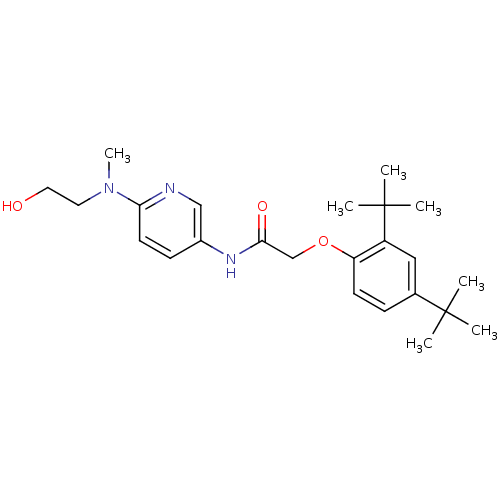
(CHEMBL2380570)Show SMILES CN(CCO)c1ccc(NC(=O)COc2ccc(cc2C(C)(C)C)C(C)(C)C)cn1 Show InChI InChI=1S/C24H35N3O3/c1-23(2,3)17-8-10-20(19(14-17)24(4,5)6)30-16-22(29)26-18-9-11-21(25-15-18)27(7)12-13-28/h8-11,14-15,28H,12-13,16H2,1-7H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154265

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-ethyl-4,5...)Show SMILES CCN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C24H26ClN5O4S2/c1-2-28-8-7-19-21(14-28)35-23(27-19)24(32)30-10-9-29(13-20(30)22(26)31)36(33,34)18-6-4-15-11-17(25)5-3-16(15)12-18/h3-6,11-12,20H,2,7-10,13-14H2,1H3,(H2,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433889
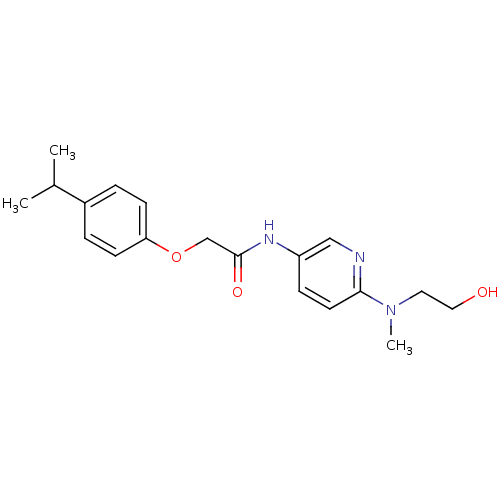
(CHEMBL2380567)Show InChI InChI=1S/C19H25N3O3/c1-14(2)15-4-7-17(8-5-15)25-13-19(24)21-16-6-9-18(20-12-16)22(3)10-11-23/h4-9,12,14,23H,10-11,13H2,1-3H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433900

(CHEMBL2380430)Show SMILES FC(F)(F)c1ccc(nc1)N1CC[C@@H](C1)NC(=O)Nc1ccccc1Br |r| Show InChI InChI=1S/C17H16BrF3N4O/c18-13-3-1-2-4-14(13)24-16(26)23-12-7-8-25(10-12)15-6-5-11(9-22-15)17(19,20)21/h1-6,9,12H,7-8,10H2,(H2,23,24,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146540
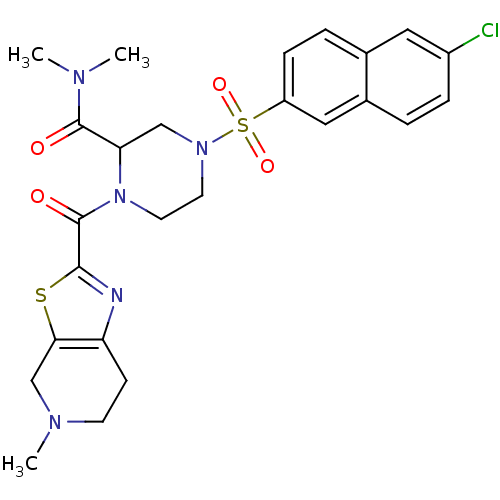
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN(C)C(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H28ClN5O4S2/c1-28(2)24(32)21-14-30(37(34,35)19-7-5-16-12-18(26)6-4-17(16)13-19)10-11-31(21)25(33)23-27-20-8-9-29(3)15-22(20)36-23/h4-7,12-13,21H,8-11,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433894
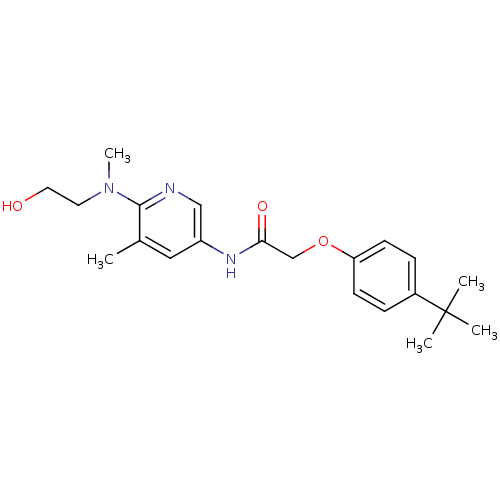
(CHEMBL2380436)Show SMILES CN(CCO)c1ncc(NC(=O)COc2ccc(cc2)C(C)(C)C)cc1C Show InChI InChI=1S/C21H29N3O3/c1-15-12-17(13-22-20(15)24(5)10-11-25)23-19(26)14-27-18-8-6-16(7-9-18)21(2,3)4/h6-9,12-13,25H,10-11,14H2,1-5H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041218

(3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-1-(1...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154270
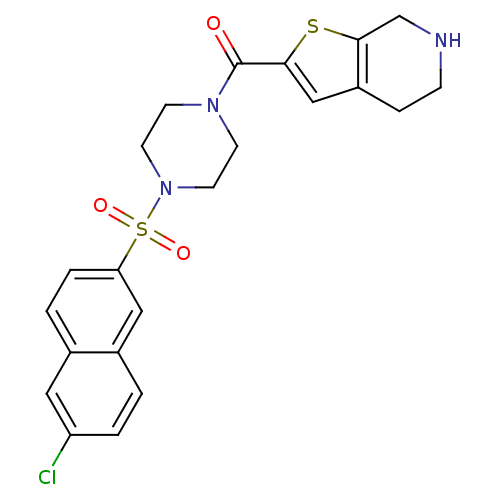
(CHEMBL182957 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1cc2CCNCc2s1 Show InChI InChI=1S/C22H22ClN3O3S2/c23-18-3-1-16-12-19(4-2-15(16)11-18)31(28,29)26-9-7-25(8-10-26)22(27)20-13-17-5-6-24-14-21(17)30-20/h1-4,11-13,24H,5-10,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154271
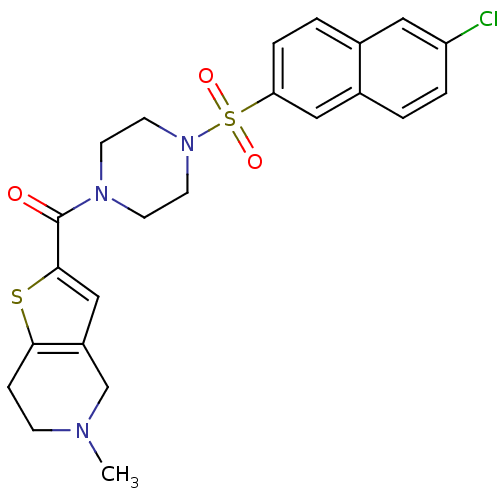
(CHEMBL185539 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2sc(cc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN3O3S2/c1-25-7-6-21-18(15-25)14-22(31-21)23(28)26-8-10-27(11-9-26)32(29,30)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
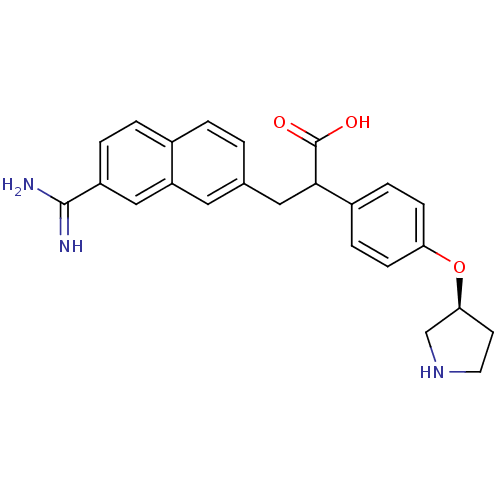
(Homo sapiens (Human)) | BDBM50041221
(3-(7-Carbamimidoyl-naphthalen-2-yl)-2-[4-((S)-pyrr...)Show SMILES NC(=N)c1ccc2ccc(CC(C(O)=O)c3ccc(O[C@H]4CCNC4)cc3)cc2c1 Show InChI InChI=1S/C24H25N3O3/c25-23(26)18-4-3-16-2-1-15(11-19(16)13-18)12-22(24(28)29)17-5-7-20(8-6-17)30-21-9-10-27-14-21/h1-8,11,13,21-22,27H,9-10,12,14H2,(H3,25,26)(H,28,29)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154266
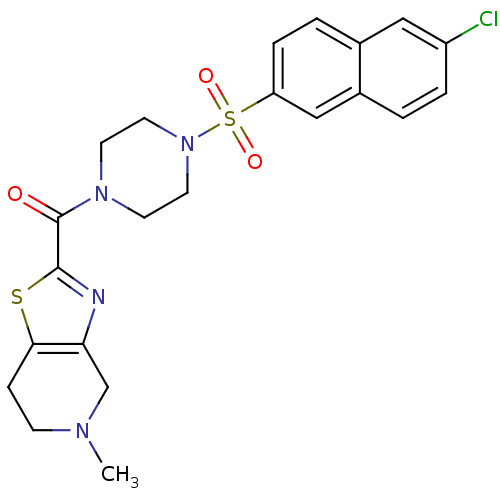
(CHEMBL181749 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2sc(nc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-20-19(14-25)24-21(31-20)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154275
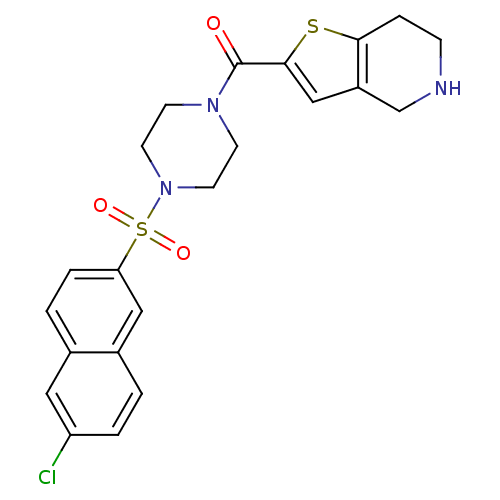
(CHEMBL183960 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1cc2CNCCc2s1 Show InChI InChI=1S/C22H22ClN3O3S2/c23-18-3-1-16-12-19(4-2-15(16)11-18)31(28,29)26-9-7-25(8-10-26)22(27)21-13-17-14-24-6-5-20(17)30-21/h1-4,11-13,24H,5-10,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041231
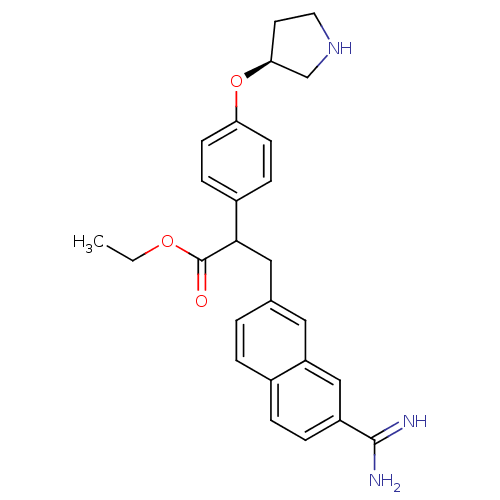
(3-(7-Carbamimidoyl-naphthalen-2-yl)-2-[4-((S)-pyrr...)Show SMILES CCOC(=O)C(Cc1ccc2ccc(cc2c1)C(N)=N)c1ccc(O[C@H]2CCNC2)cc1 Show InChI InChI=1S/C26H29N3O3/c1-2-31-26(30)24(19-7-9-22(10-8-19)32-23-11-12-29-16-23)14-17-3-4-18-5-6-20(25(27)28)15-21(18)13-17/h3-10,13,15,23-24,29H,2,11-12,14,16H2,1H3,(H3,27,28)/t23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154273
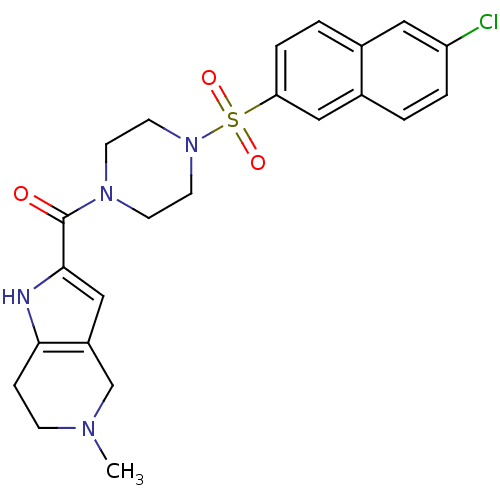
(CHEMBL365826 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2[nH]c(cc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H25ClN4O3S/c1-26-7-6-21-18(15-26)14-22(25-21)23(29)27-8-10-28(11-9-27)32(30,31)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14,25H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041219
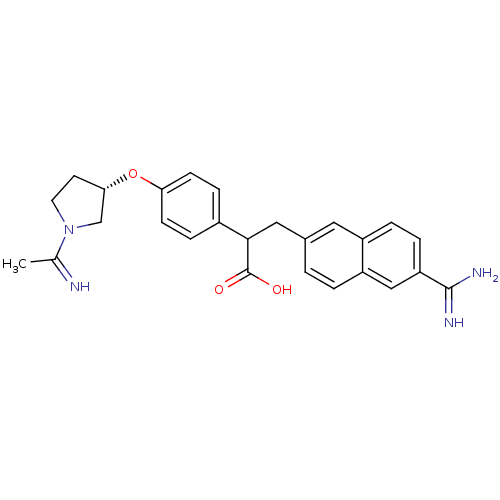
(3-(6-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-1-(1...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Cc1ccc2cc(ccc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-18(7-9-22)24(26(31)32)13-17-2-3-20-14-21(25(28)29)5-4-19(20)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433899
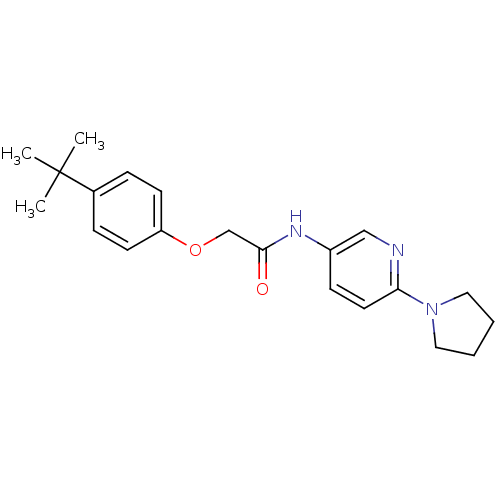
(CHEMBL2380431)Show SMILES CC(C)(C)c1ccc(OCC(=O)Nc2ccc(nc2)N2CCCC2)cc1 Show InChI InChI=1S/C21H27N3O2/c1-21(2,3)16-6-9-18(10-7-16)26-15-20(25)23-17-8-11-19(22-14-17)24-12-4-5-13-24/h6-11,14H,4-5,12-13,15H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041217
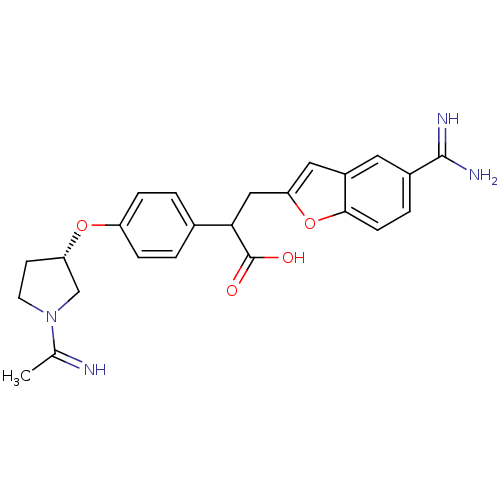
(3-(5-Carbamimidoyl-benzofuran-2-yl)-2-{4-[(S)-1-(1...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Cc1cc2cc(ccc2o1)C(N)=N)C(O)=O Show InChI InChI=1S/C24H26N4O4/c1-14(25)28-9-8-19(13-28)31-18-5-2-15(3-6-18)21(24(29)30)12-20-11-17-10-16(23(26)27)4-7-22(17)32-20/h2-7,10-11,19,21,25H,8-9,12-13H2,1H3,(H3,26,27)(H,29,30)/t19-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
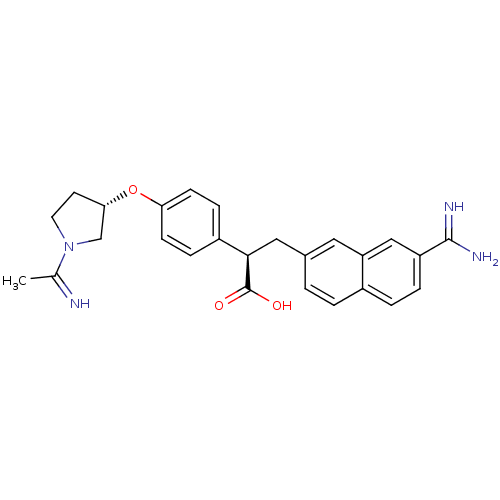
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041224
((R)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433898
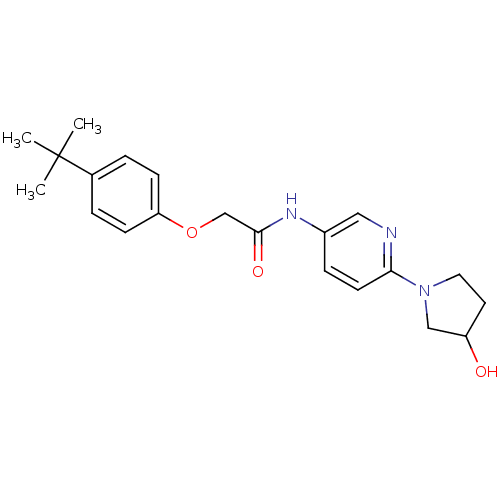
(CHEMBL2380432)Show SMILES CC(C)(C)c1ccc(OCC(=O)Nc2ccc(nc2)N2CCC(O)C2)cc1 Show InChI InChI=1S/C21H27N3O3/c1-21(2,3)15-4-7-18(8-5-15)27-14-20(26)23-16-6-9-19(22-12-16)24-11-10-17(25)13-24/h4-9,12,17,25H,10-11,13-14H2,1-3H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 516 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154262
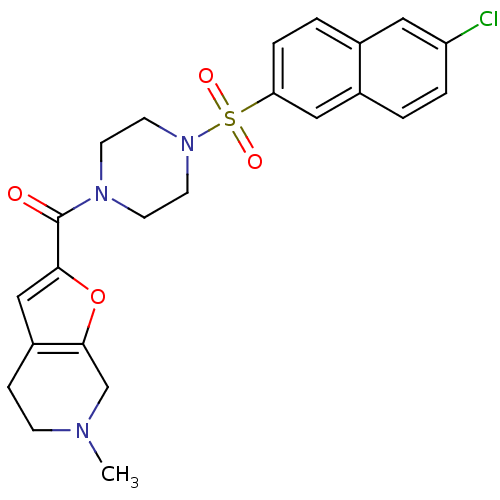
(CHEMBL185141 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2cc(oc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN3O4S/c1-25-7-6-18-14-21(31-22(18)15-25)23(28)26-8-10-27(11-9-26)32(29,30)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433896
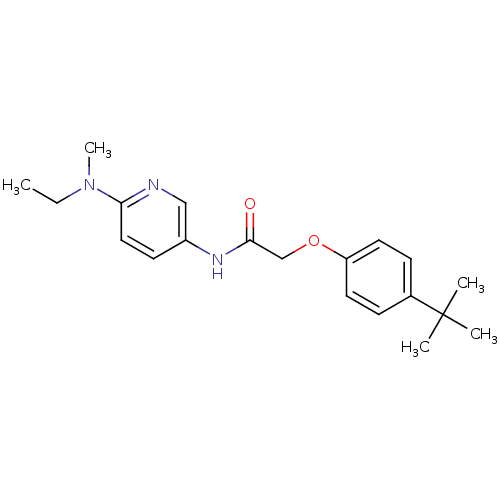
(CHEMBL2380434)Show InChI InChI=1S/C20H27N3O2/c1-6-23(5)18-12-9-16(13-21-18)22-19(24)14-25-17-10-7-15(8-11-17)20(2,3)4/h7-13H,6,14H2,1-5H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 583 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154268
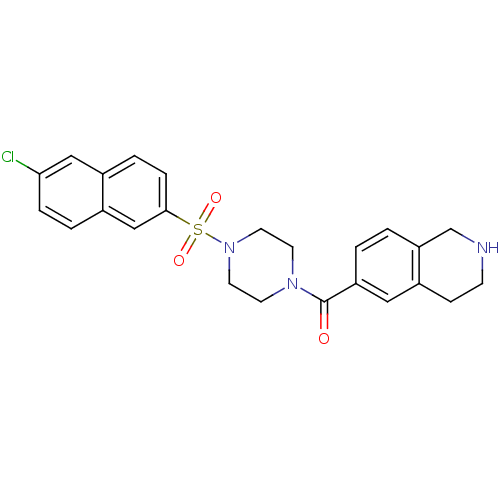
(CHEMBL363048 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc2CNCCc2c1 Show InChI InChI=1S/C24H24ClN3O3S/c25-22-5-3-18-15-23(6-4-17(18)14-22)32(30,31)28-11-9-27(10-12-28)24(29)20-1-2-21-16-26-8-7-19(21)13-20/h1-6,13-15,26H,7-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041216
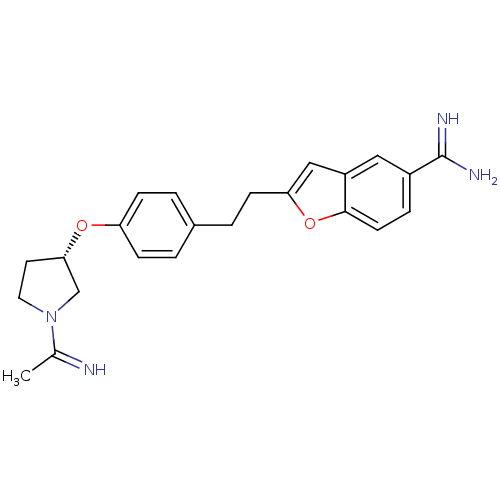
(2-(2-{4-[(S)-1-(1-Imino-ethyl)-pyrrolidin-3-yloxy]...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(CCc2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C23H26N4O2/c1-15(24)27-11-10-21(14-27)28-19-6-2-16(3-7-19)4-8-20-13-18-12-17(23(25)26)5-9-22(18)29-20/h2-3,5-7,9,12-13,21,24H,4,8,10-11,14H2,1H3,(H3,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146533

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCc1ccccc1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C30H30ClN5O4S2/c1-34-12-11-25-27(19-34)41-29(33-25)30(38)36-14-13-35(18-26(36)28(37)32-17-20-5-3-2-4-6-20)42(39,40)24-10-8-21-15-23(31)9-7-22(21)16-24/h2-10,15-16,26H,11-14,17-19H2,1H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
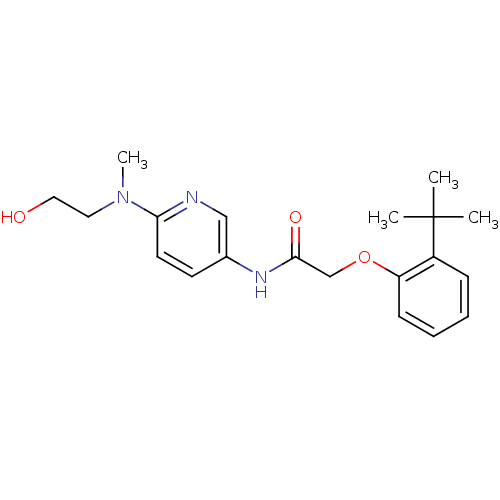
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50433891
(CHEMBL2380565)Show InChI InChI=1S/C20H27N3O3/c1-20(2,3)16-7-5-6-8-17(16)26-14-19(25)22-15-9-10-18(21-13-15)23(4)11-12-24/h5-10,13,24H,11-12,14H2,1-4H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 796 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 transfected in CHOK1 cells assessed as inhibition of capsaicin-induced intracellular calcium level measured for 12... |
Bioorg Med Chem Lett 23: 3154-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.016
BindingDB Entry DOI: 10.7270/Q2X63PBZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146537

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CC(C)NC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C26H30ClN5O4S2/c1-16(2)28-24(33)22-14-31(38(35,36)20-7-5-17-12-19(27)6-4-18(17)13-20)10-11-32(22)26(34)25-29-21-8-9-30(3)15-23(21)37-25/h4-7,12-13,16,22H,8-11,14-15H2,1-3H3,(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041223
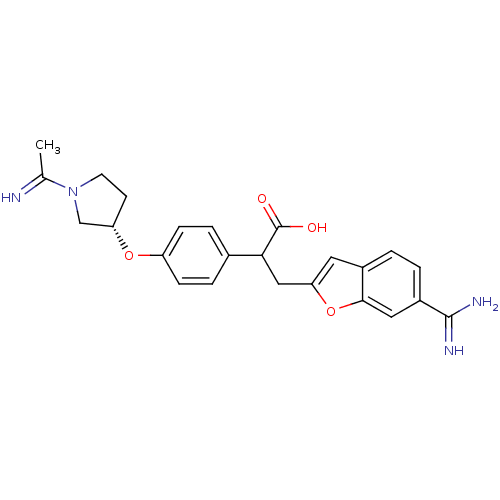
(3-(6-Carbamimidoyl-benzofuran-2-yl)-2-{4-[(S)-1-(1...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Cc1cc2ccc(cc2o1)C(N)=N)C(O)=O Show InChI InChI=1S/C24H26N4O4/c1-14(25)28-9-8-19(13-28)31-18-6-4-15(5-7-18)21(24(29)30)12-20-10-16-2-3-17(23(26)27)11-22(16)32-20/h2-7,10-11,19,21,25H,8-9,12-13H2,1H3,(H3,26,27)(H,29,30)/t19-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
In vitro evaluation for the concentration needed for inhibiting factor Xa by 50% |
J Med Chem 37: 1200-7 (1994)
BindingDB Entry DOI: 10.7270/Q2FF3RF6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data