 Found 252 hits with Last Name = 'martinot' and Initial = 'ta'
Found 252 hits with Last Name = 'martinot' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
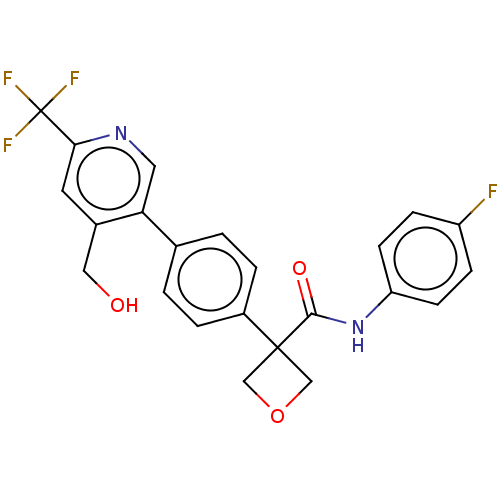
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
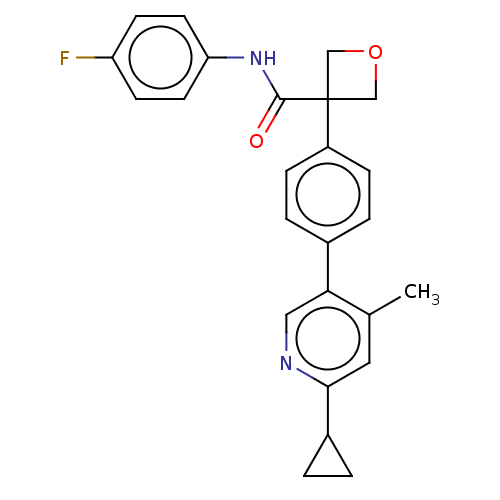
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
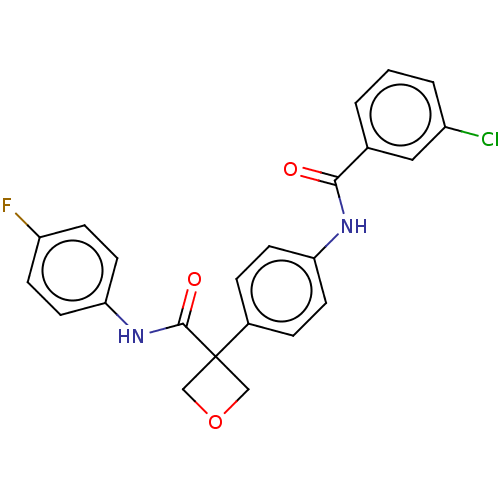
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609728

(CHEMBL5267350)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1n[nH]c2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742
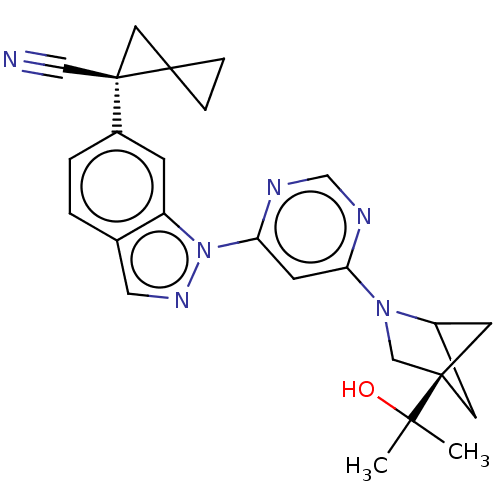
(CHEMBL5286106)Show SMILES CC(C)(O)[C@]12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,wD:4.3,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.67,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.85,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742

(CHEMBL5286106)Show SMILES CC(C)(O)[C@]12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,wD:4.3,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.67,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.85,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609741

(CHEMBL5284341)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;4.54,-2.51,;4.14,-4,;5.68,-3.91,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207
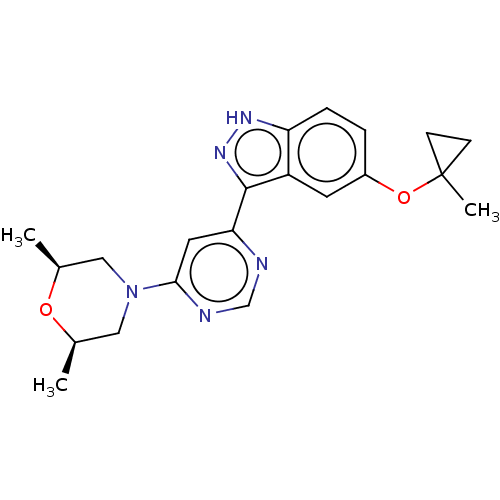
(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609741

(CHEMBL5284341)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;4.54,-2.51,;4.14,-4,;5.68,-3.91,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552941

(CHEMBL4764710)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025
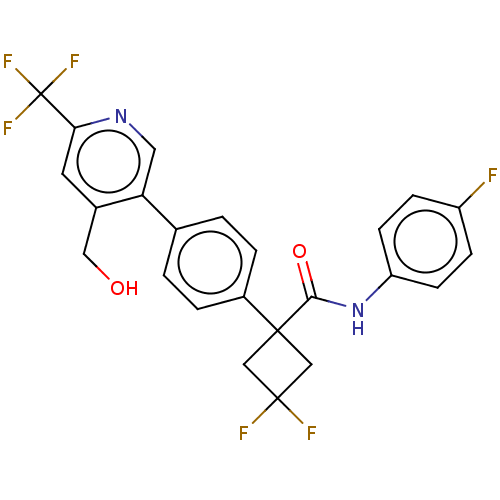
(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742

(CHEMBL5286106)Show SMILES CC(C)(O)[C@]12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,wD:4.3,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.67,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.85,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573261

(CHEMBL4862297)Show SMILES C[C@H]1C[C@@]1(NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573260
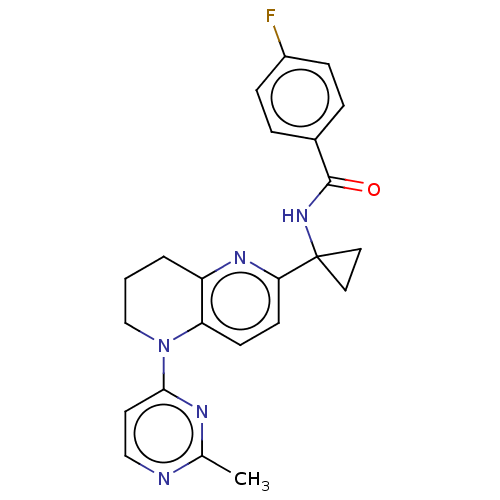
(CHEMBL4859249)Show SMILES Cc1nccc(n1)N1CCCc2nc(ccc12)C1(CC1)NC(=O)c1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604024

(CHEMBL5193283)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CCC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207

(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573259
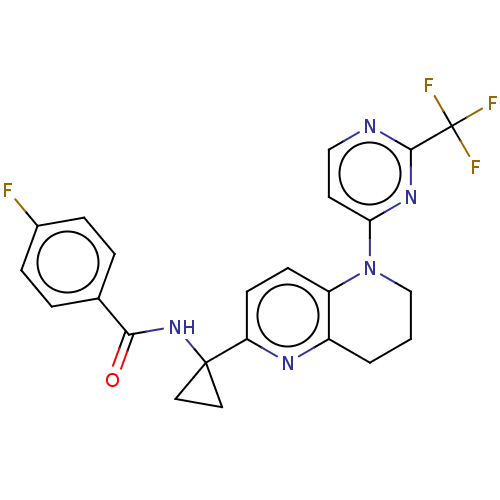
(CHEMBL4847724)Show SMILES Fc1ccc(cc1)C(=O)NC1(CC1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552934

(CHEMBL4744727)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025

(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552931

(CHEMBL4755227)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579614

(CHEMBL4871970)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)CCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552948

(CHEMBL4779920)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccncn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573262

(CHEMBL4870929)Show SMILES [H][C@@]12C[C@]1([H])c1nc(ccc1N(C2)c1ccnc(C)n1)C1(CC1)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609738
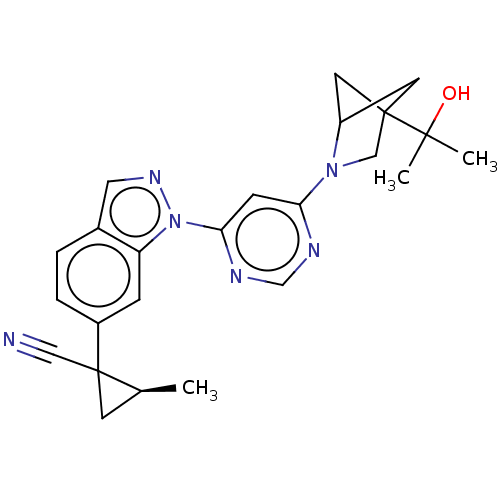
(CHEMBL5267690)Show SMILES C[C@H]1CC1(C#N)c1ccc2cnn(-c3cc(ncn3)N3CC4(CC3C4)C(C)(C)O)c2c1 |r,wU:1.0,(-3.54,-1.2,;-4.87,-.43,;-6.42,-.43,;-5.64,.91,;-6.98,1.68,;-8.31,2.45,;-4.31,1.68,;-4.31,3.23,;-2.98,4,;-1.65,3.23,;-.18,3.7,;.72,2.46,;-.18,1.21,;.22,-.28,;1.7,-.68,;2.1,-2.16,;1.01,-3.25,;-.47,-2.86,;-.88,-1.37,;3.59,-2.56,;4.78,-1.59,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;6.77,-1.06,;6.77,.49,;5.44,-.29,;8.31,-.98,;-1.65,1.69,;-2.97,.92,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609738

(CHEMBL5267690)Show SMILES C[C@H]1CC1(C#N)c1ccc2cnn(-c3cc(ncn3)N3CC4(CC3C4)C(C)(C)O)c2c1 |r,wU:1.0,(-3.54,-1.2,;-4.87,-.43,;-6.42,-.43,;-5.64,.91,;-6.98,1.68,;-8.31,2.45,;-4.31,1.68,;-4.31,3.23,;-2.98,4,;-1.65,3.23,;-.18,3.7,;.72,2.46,;-.18,1.21,;.22,-.28,;1.7,-.68,;2.1,-2.16,;1.01,-3.25,;-.47,-2.86,;-.88,-1.37,;3.59,-2.56,;4.78,-1.59,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;6.77,-1.06,;6.77,.49,;5.44,-.29,;8.31,-.98,;-1.65,1.69,;-2.97,.92,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023

(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503

(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604022
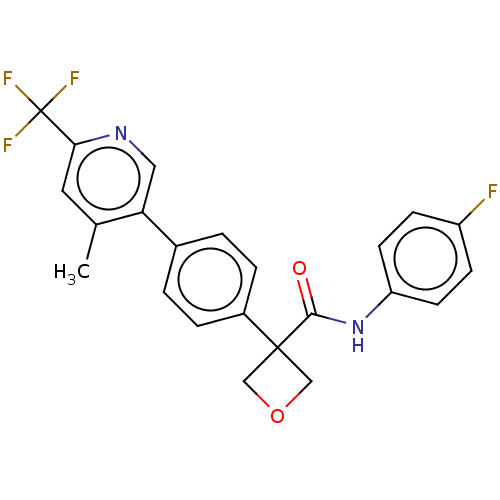
(CHEMBL5180607)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579610

(CHEMBL4873520)Show SMILES [H][C@]12CN[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:9:8:7.6.5:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021

(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023

(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552940

(CHEMBL4749009)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609729

(CHEMBL5290520)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1ncn2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604024

(CHEMBL5193283)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CCC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552937

(CHEMBL4788473)Show SMILES CCOC(=O)N1CCCc2nc(ccc12)[C@@H](C)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552930
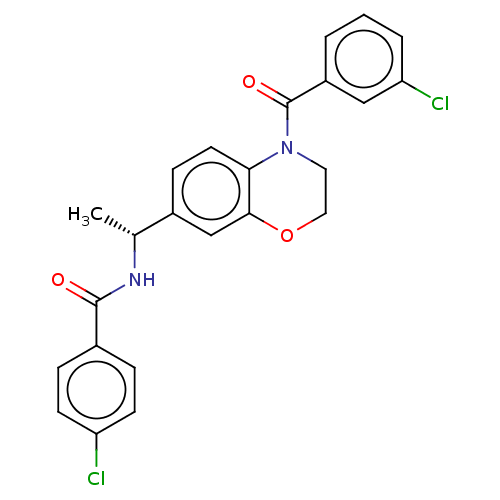
(CHEMBL4744926)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCOc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579616

(CHEMBL4845730)Show SMILES [H][C@]12CN[C@H](C(=O)OC)[C@@]1(CCCB(O)O)CCCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552949

(CHEMBL4779248)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552949

(CHEMBL4779248)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579611
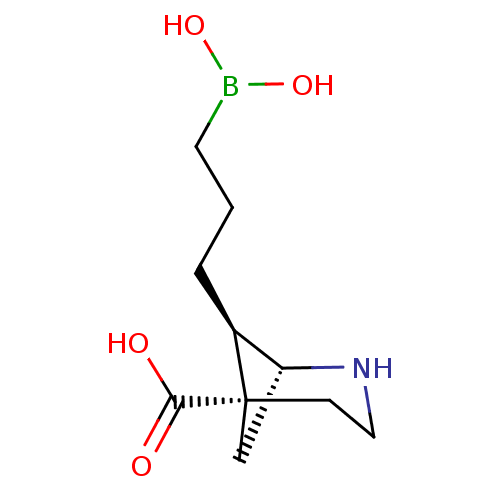
(CHEMBL4871097)Show SMILES [H][C@]12C[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:8:7:2:4.5.6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609734
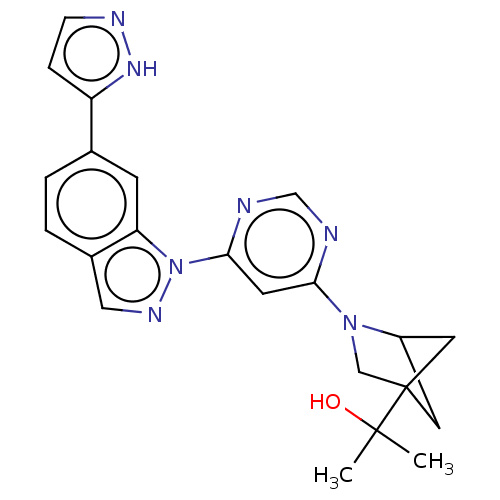
(CHEMBL5279615)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |(8.37,-.94,;7.28,-2.03,;6.88,-.54,;8.37,-3.12,;5.79,-2.43,;5.39,-3.92,;3.85,-4,;4.42,-2.54,;3.3,-2.56,;4.5,-1.59,;1.81,-2.16,;1.42,-.68,;-.07,-.28,;-1.16,-1.37,;-.76,-2.86,;.72,-3.25,;-.47,1.21,;.44,2.46,;-.47,3.7,;-1.93,3.23,;-3.26,4,;-4.6,3.23,;-4.6,1.68,;-3.26,.92,;-1.93,1.69,;-5.93,.91,;-7.34,1.54,;-8.37,.39,;-7.6,-.94,;-6.09,-.62,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609728

(CHEMBL5267350)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1n[nH]c2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538536
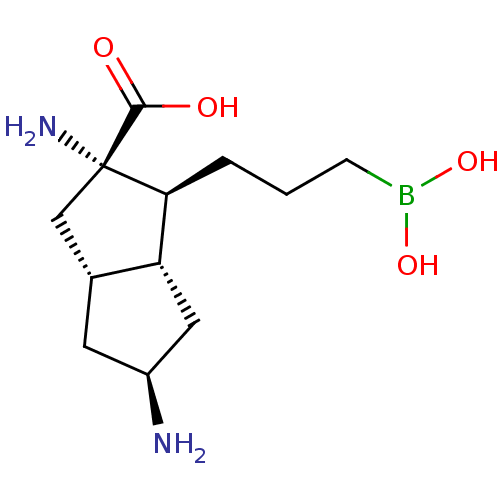
(CHEMBL4639893)Show SMILES [H][C@]12C[C@H](N)C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C12H23BN2O4/c14-8-4-7-6-12(15,11(16)17)10(9(7)5-8)2-1-3-13(18)19/h7-10,18-19H,1-6,14-15H2,(H,16,17)/t7-,8+,9-,10+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579615

(CHEMBL4865209)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)COC2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538548
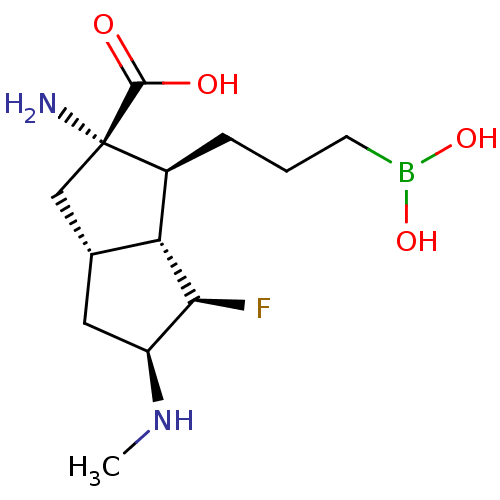
(CHEMBL4640681)Show SMILES [H][C@]12C[C@H](NC)[C@H](F)[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C13H24BFN2O4/c1-17-9-5-7-6-13(16,12(18)19)8(10(7)11(9)15)3-2-4-14(20)21/h7-11,17,20-21H,2-6,16H2,1H3,(H,18,19)/t7-,8+,9+,10-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data