

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38045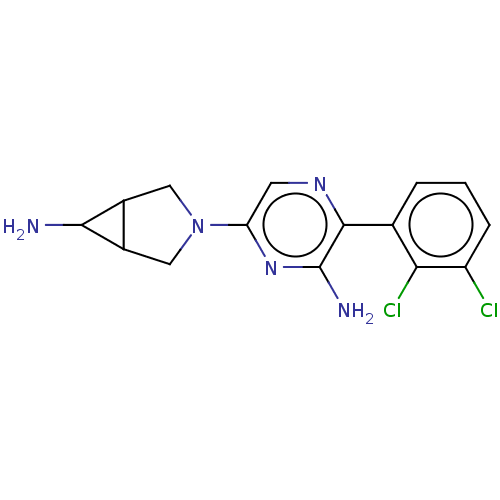 (US10093646, Example 13 | US10774065, Example 13 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38042 (US10093646, Example 12 | US10774065, Example 12 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38042 (US10093646, Example 12 | US10774065, Example 12 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38045 (US10093646, Example 13 | US10774065, Example 13 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38042 (US10093646, Example 12 | US10774065, Example 12 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38045 (US10093646, Example 13 | US10774065, Example 13 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38048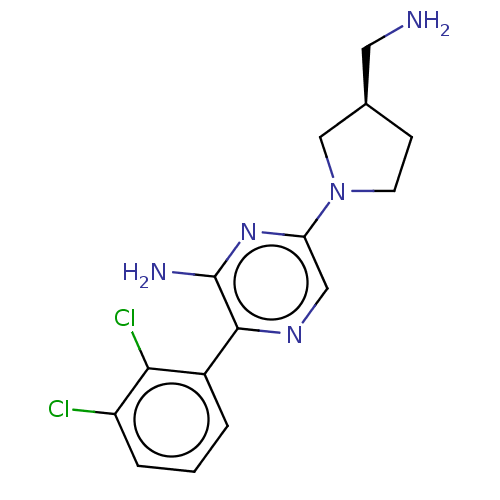 (US10093646, Example 14 | US10774065, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38048 (US10093646, Example 14 | US10774065, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38048 (US10093646, Example 14 | US10774065, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38019 (US10093646, Compound 1 | US10301278, Example 00003...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38019 (US10093646, Compound 1 | US10301278, Example 00003...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38019 (US10093646, Compound 1 | US10301278, Example 00003...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38067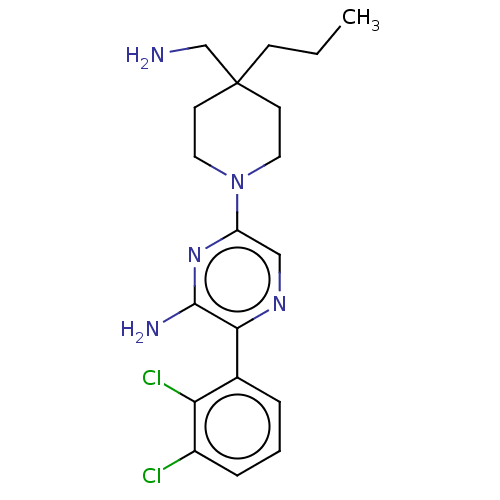 (US10093646, Example 15 | US10774065, Example 15 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38067 (US10093646, Example 15 | US10774065, Example 15 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38067 (US10093646, Example 15 | US10774065, Example 15 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38027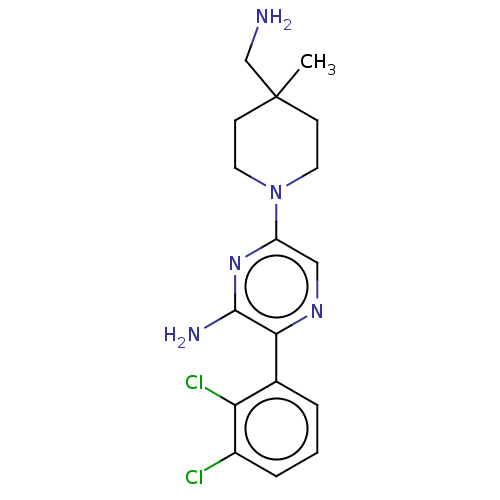 (US10093646, Example 8 | US10774065, Example 8 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38027 (US10093646, Example 8 | US10774065, Example 8 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38027 (US10093646, Example 8 | US10774065, Example 8 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM461308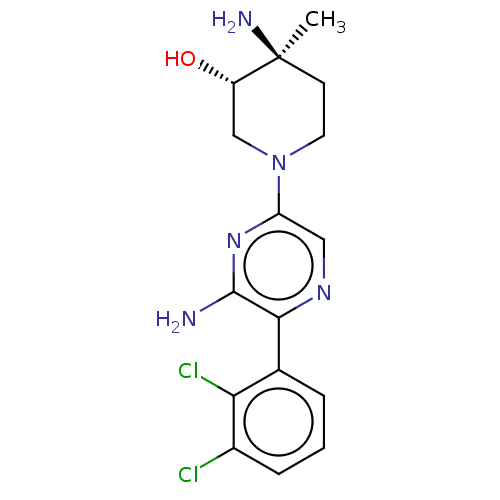 (US10774065, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38073 (US10093646, Example 18 | US10774065, Example 18 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38069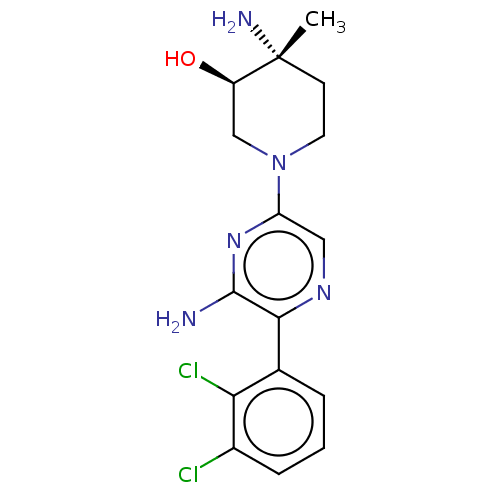 (US10093646, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Novartis AG US Patent | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q25X2C0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38073 (US10093646, Example 18 | US10774065, Example 18 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM562187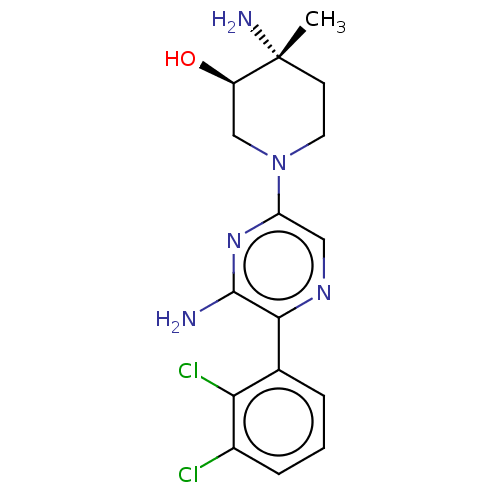 (US11401259, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS5ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM38073 (US10093646, Example 18 | US10774065, Example 18 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | US Patent US10774065 (2020) BindingDB Entry DOI: 10.7270/Q2WW7MS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38150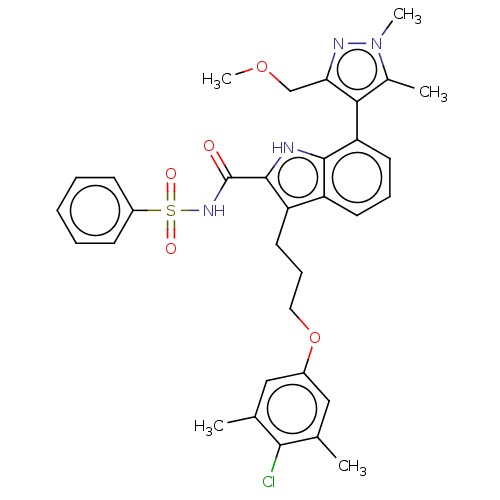 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(3-(m...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289084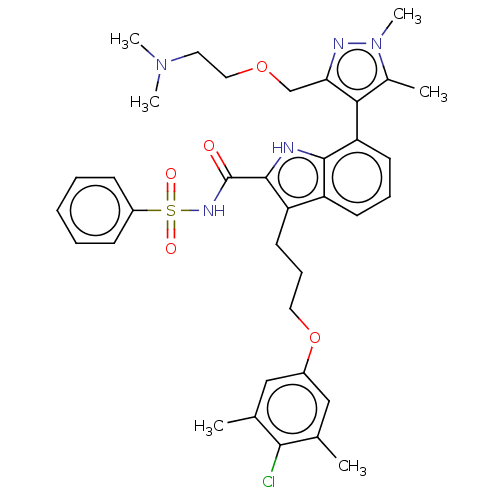 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(3-((...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289083 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(1,5-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289082 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(1,5-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50160010 (CHEMBL3786147 | US10093640, Example 305 | US108440...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289080 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(1-is...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289079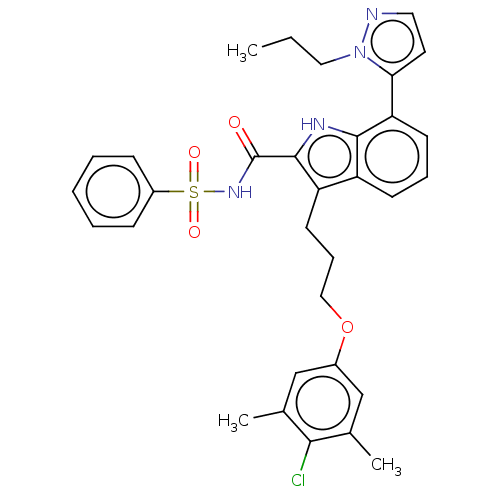 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-(phen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289078 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(1-et...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289076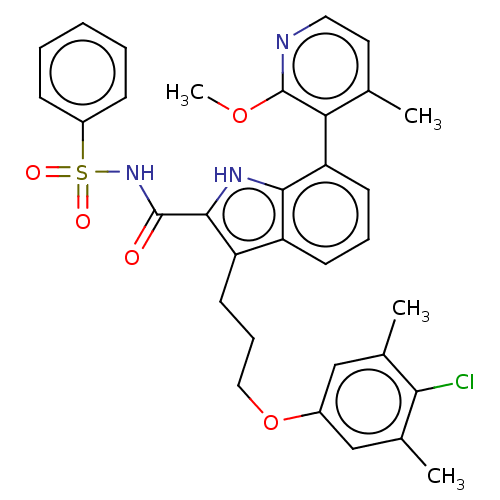 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(2-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM289075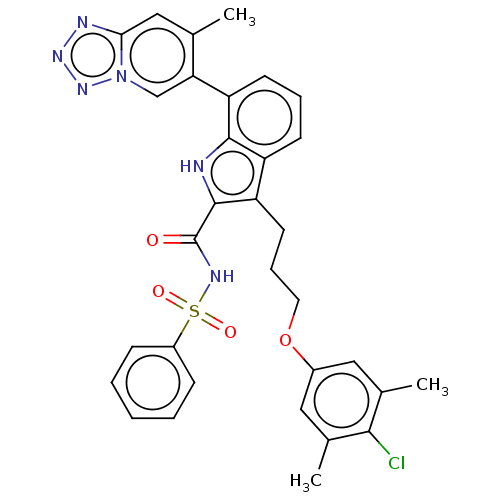 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(7-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38065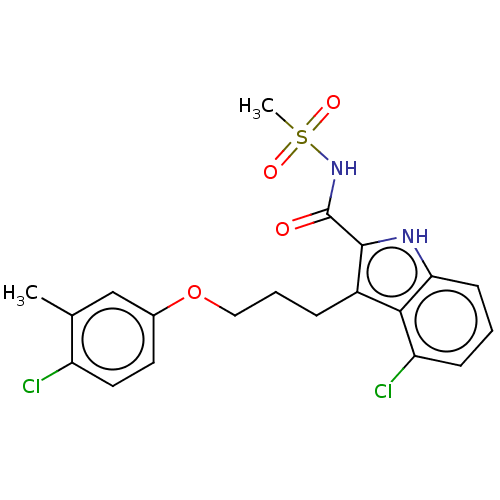 (4-chloro-3-(3-(4-chloro-3-methylphenoxy)propyl)-N-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38066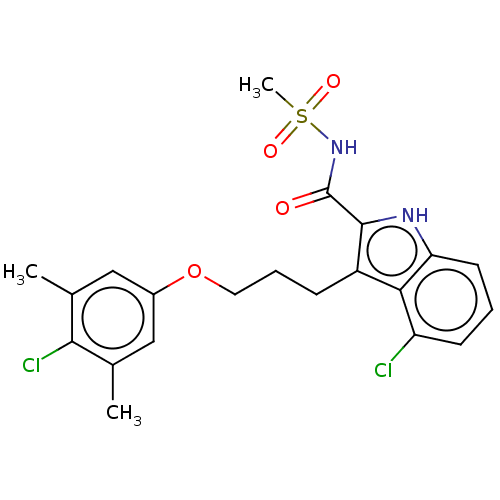 (4-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38101 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(3,5-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50160000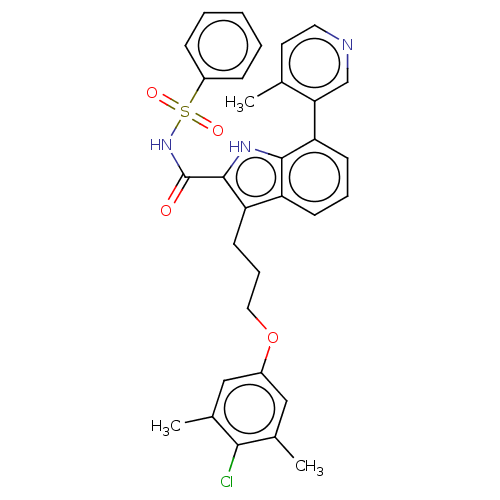 (CHEMBL3786018 | US10093640, Example 89 | US1084403...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38103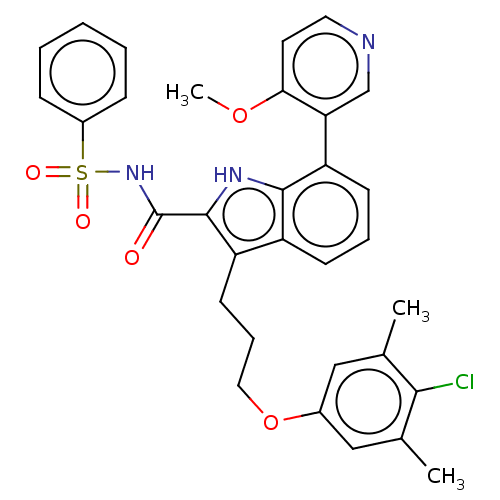 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(4-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38104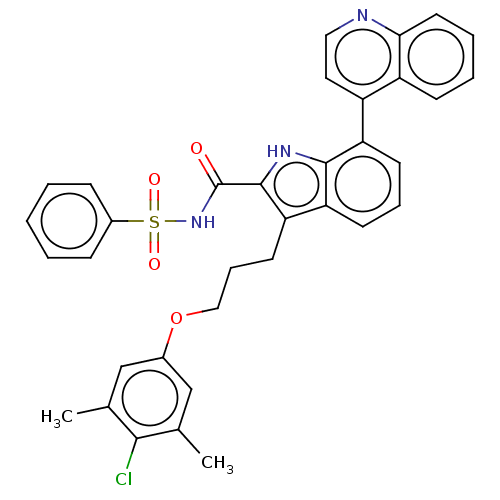 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-(phen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38109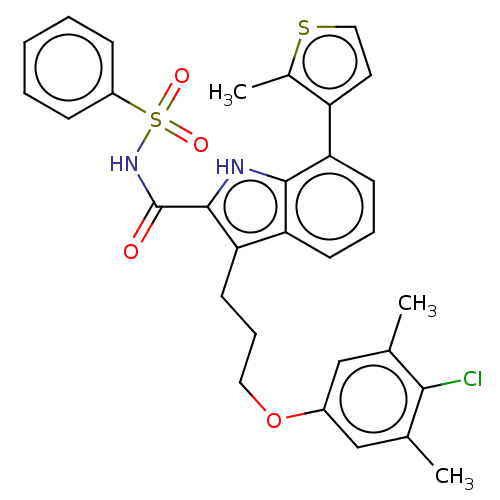 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(2-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38110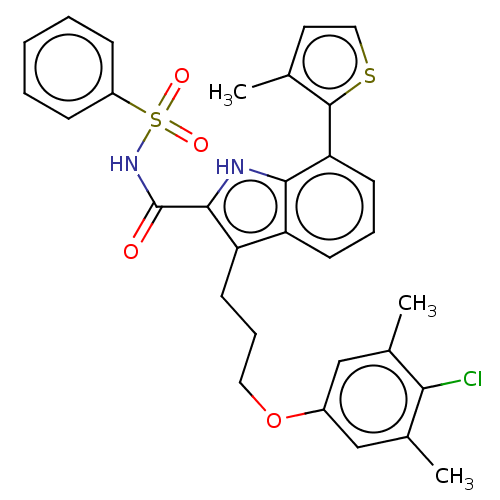 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(3-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38112 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(1-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38114 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-(1-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38115 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-(phen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38119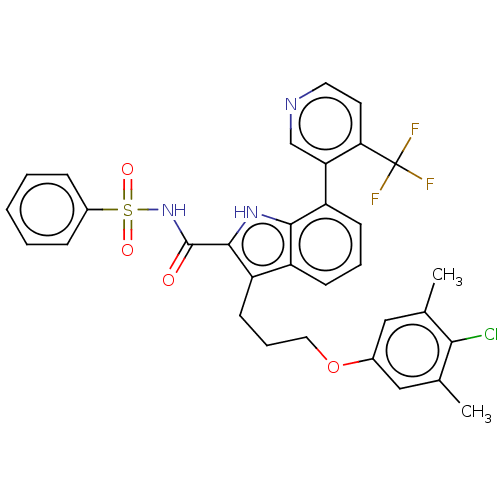 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-(phen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38120 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-(phen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38131 (4-bromo-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38134 (3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-methy...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM38141 (6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Compound affinity was measured using a fluorescence polarization anisotropy competition assay. Anisotropy measurements were carried out in 384-well, ... | US Patent US10093646 (2018) BindingDB Entry DOI: 10.7270/Q29P33PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 352 total ) | Next | Last >> |