

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Rattus norvegicus) | BDBM50364076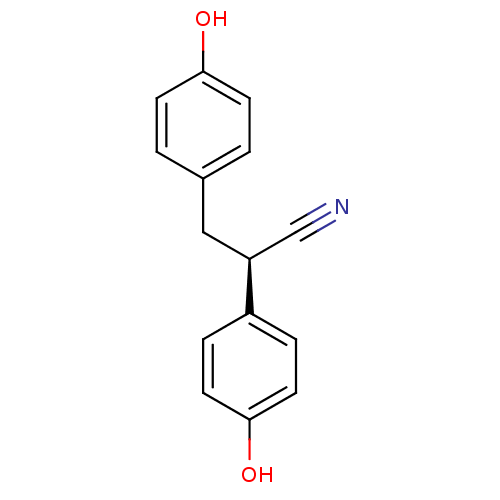 (CHEMBL198159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Displacement of [3H]E2 from rat ERbeta1 after 90 mins | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM50364075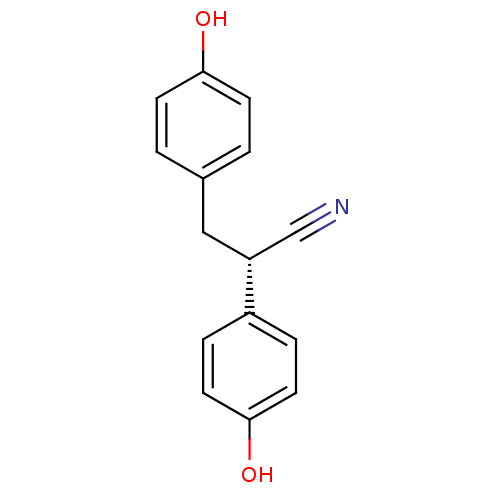 (CHEMBL1950809) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Displacement of [3H]E2 from rat ERbeta1 after 90 mins | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50410785 (CHEMBL5291155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50410784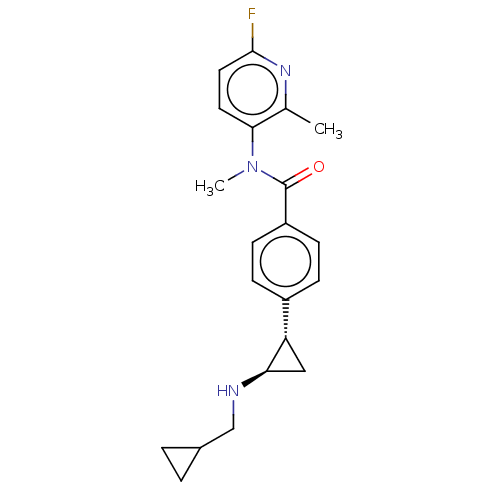 (CHEMBL5280566) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50410789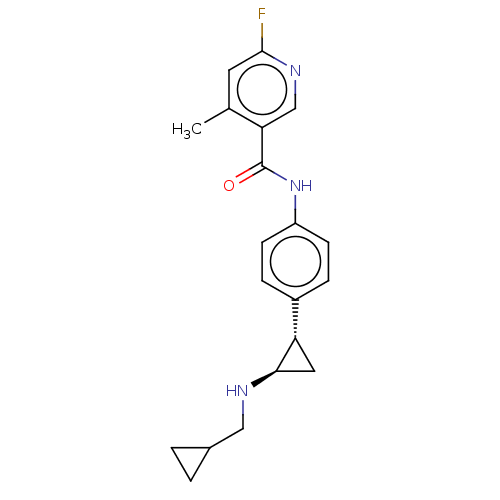 (CHEMBL5277056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50410788 (CHEMBL5281376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50410786 (CHEMBL5270114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50410787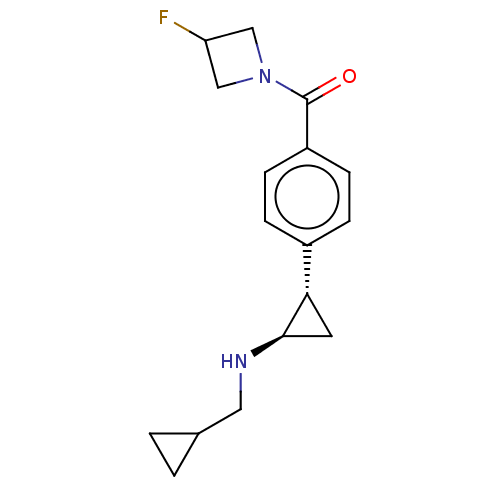 (CHEMBL5270703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50410789 (CHEMBL5277056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50410789 (CHEMBL5277056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against FAAH in Wistar rat brain homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635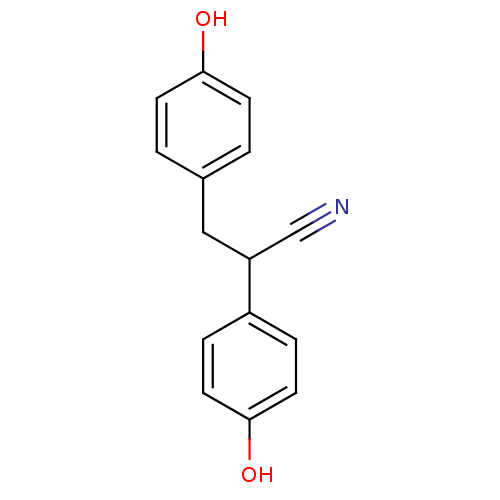 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERalpha ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitme... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 637 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERalpha ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitme... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 876 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERalpha ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitme... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERalpha ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitme... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 32.3 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERbeta ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitmen... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERbeta ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitmen... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 16.7 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERbeta ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitmen... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled ERbeta ligand binding domain expressed in Escherichia coli BL21 cells assessed as recruitmen... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERalpha ligand binding domain expressed in Escherichia coli BL21 cells after 1 ... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERalpha ligand binding domain expressed in Escherichia coli BL21 cells after 1 ... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERalpha ligand binding domain expressed in Escherichia coli BL21 cells after 1 ... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERalpha ligand binding domain expressed in Escherichia coli BL21 cells after 1 ... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERbeta ligand binding domain expressed in Escherichia coli BL21 cells after 1 h... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERbeta ligand binding domain expressed in Escherichia coli BL21 cells after 1 h... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERbeta ligand binding domain expressed in Escherichia coli BL21 cells after 1 h... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human N-His6-tagged terbium-labelled NRID-SRC3 of ERbeta ligand binding domain expressed in Escherichia coli BL21 cells after 1 h... | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 205 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 212 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 304 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 286 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 317 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364076 (CHEMBL198159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50364075 (CHEMBL1950809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 208 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||