

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227363 (CHEMBL299837) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227366 (CHEMBL48927) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227364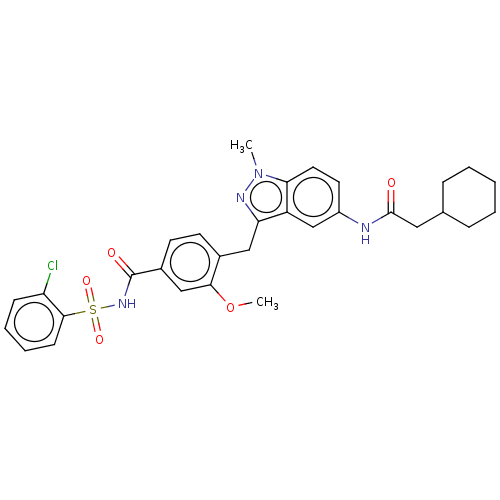 (CHEMBL299093) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227361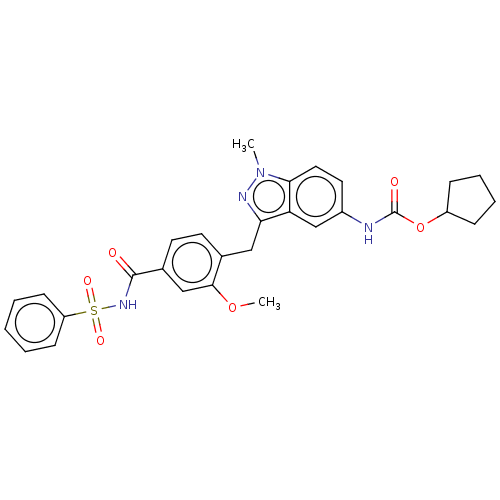 (CHEMBL51585) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015528 (CHEMBL50370 | N-[3-(4-Benzenesulfonylaminocarbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227365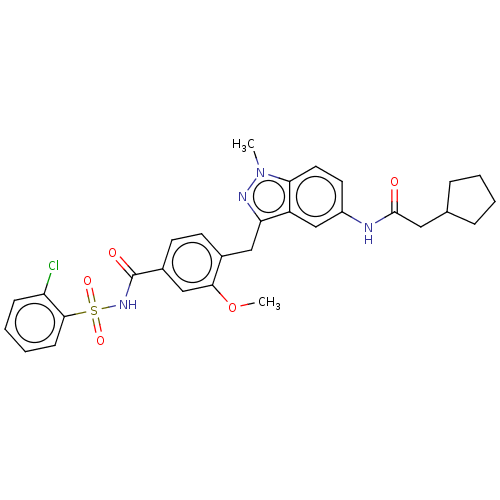 (CHEMBL300096) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227640 (CHEMBL297952) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227642 (CHEMBL50562) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227355 (CHEMBL296821) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227356 (CHEMBL49944) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227358 (CHEMBL412056) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075926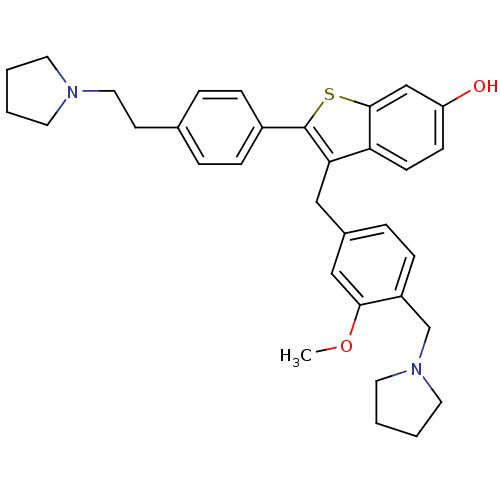 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463404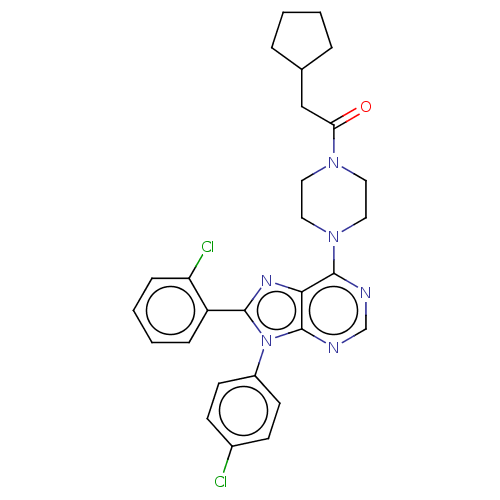 (CHEMBL4244751) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075934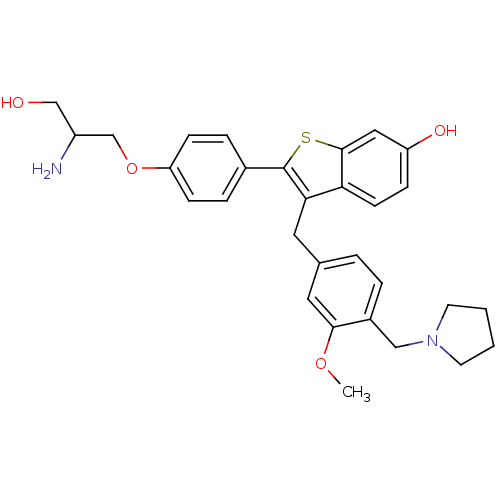 (2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50000819 (2-Cyclopentyl-N-{3-[2-methoxy-4-(toluene-2-sulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227390 (CHEMBL301616) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015523 (CHEMBL52492 | N-{4-[5-(3-Cyclopentyl-ureido)-1-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075928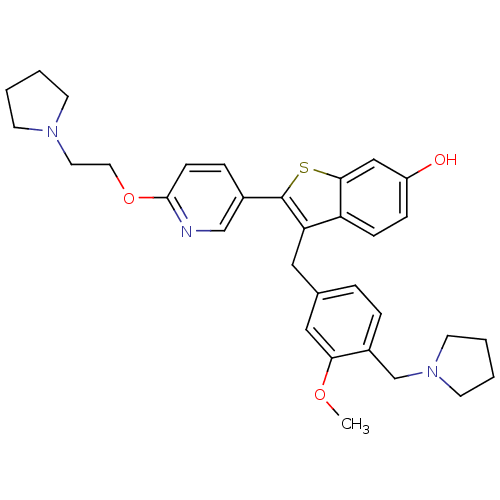 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075937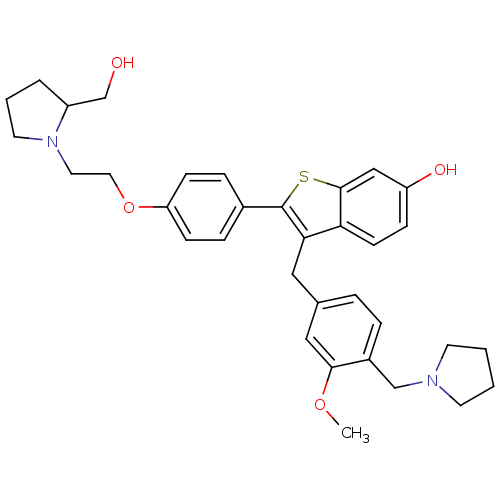 (2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463413 (CHEMBL4242477) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27337 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227389 (CHEMBL51580) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015551 (CHEMBL301498 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227367 (CHEMBL48435) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075932 (2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461710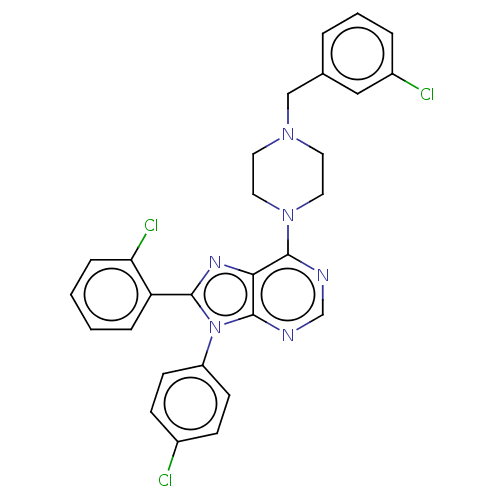 (CHEMBL4225147) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075938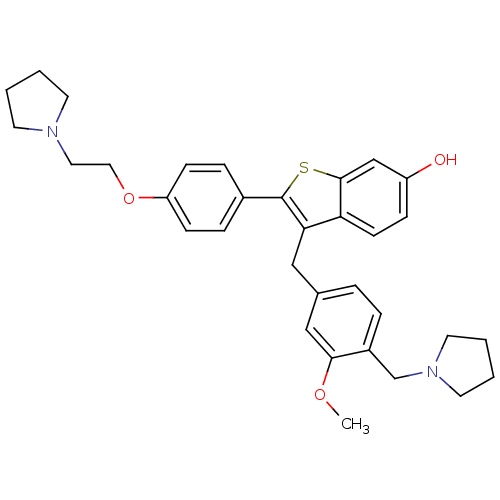 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015517 (2-Cyclopentyl-N-{3-[2-methoxy-4-(toluene-2-sulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463410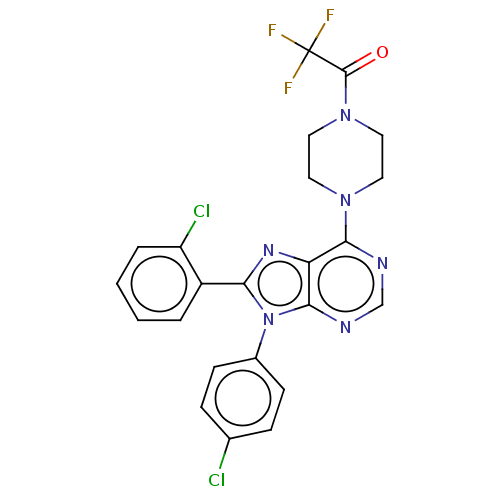 (CHEMBL4250064) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461703 (CHEMBL4225421) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461698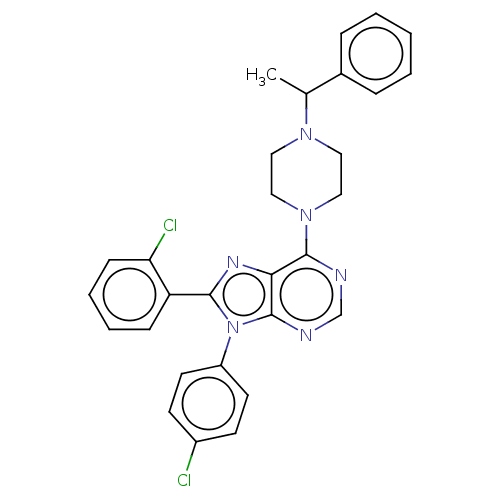 (CHEMBL4227354) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463403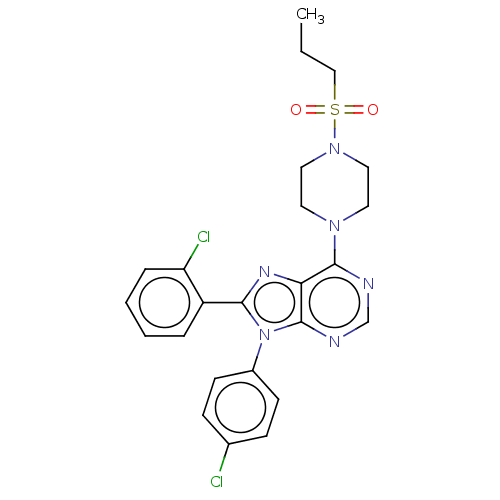 (CHEMBL4248795) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463398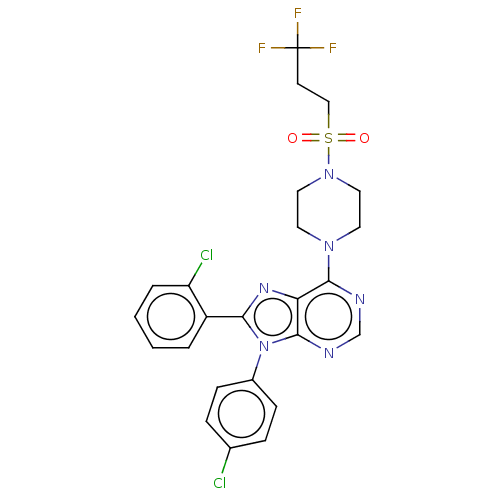 (CHEMBL4238756) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463414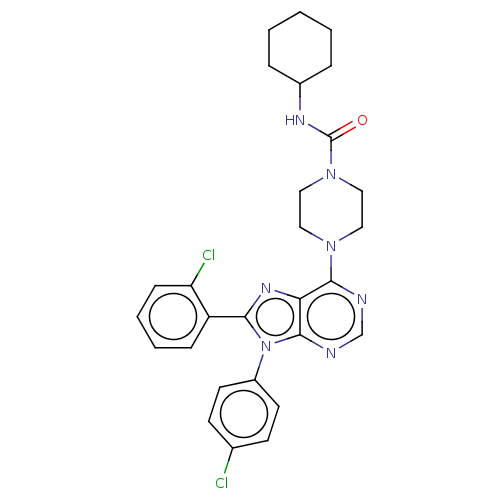 (CHEMBL4246283) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227638 (CHEMBL49566) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015540 (CHEMBL431348 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463417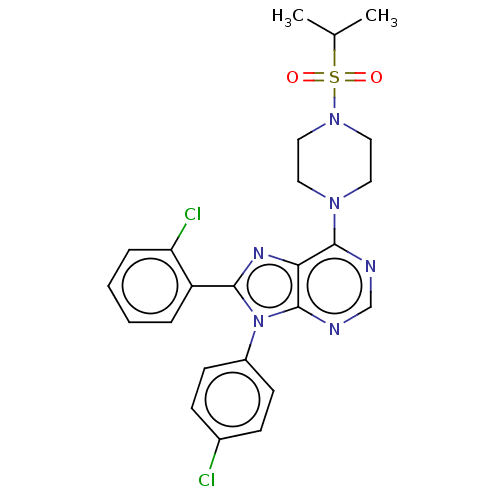 (CHEMBL4244992) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463419 (CHEMBL4244624) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075935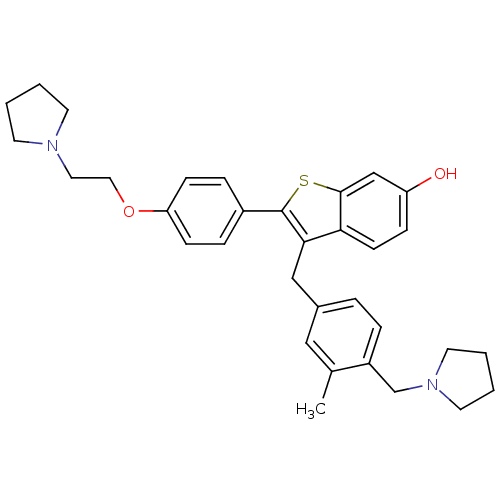 (3-(3-Methyl-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461691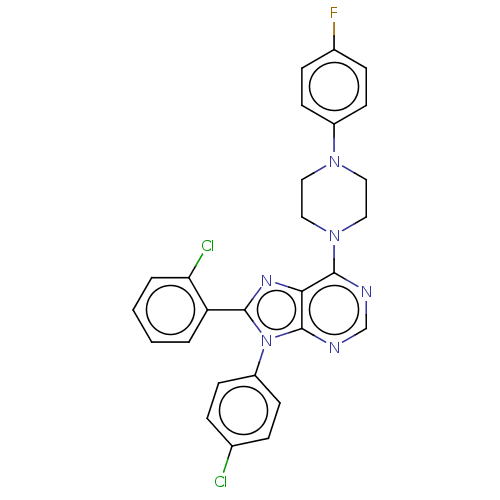 (CHEMBL4224946) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461712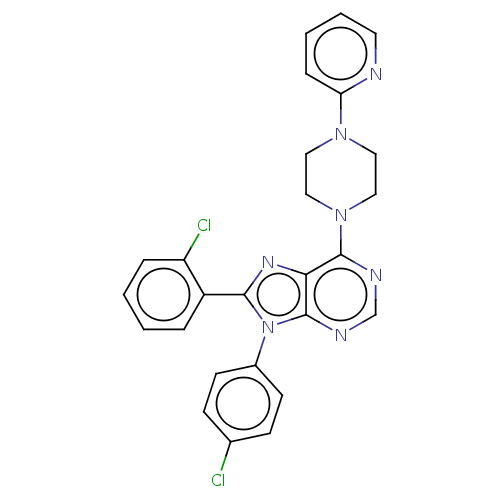 (CHEMBL4229172) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463422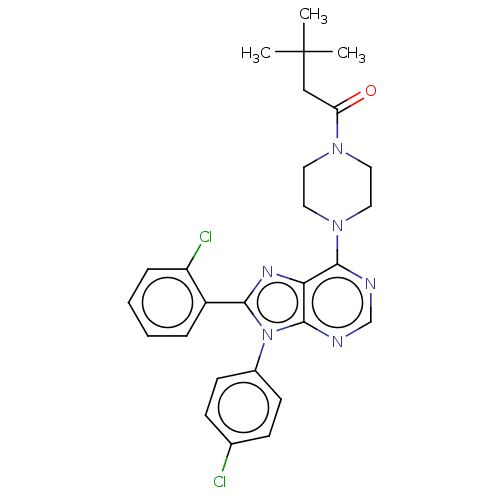 (CHEMBL4248778) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463418 (CHEMBL4238314) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461705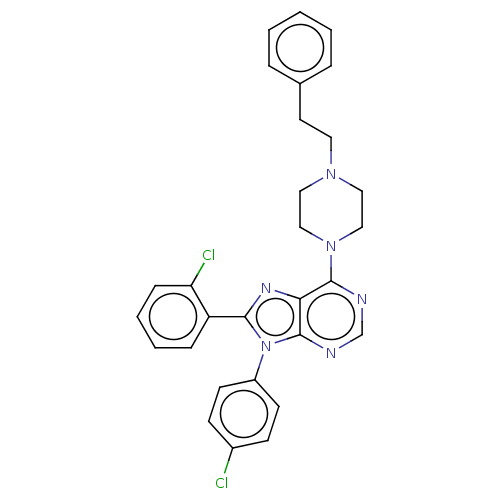 (CHEMBL4226777) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461688 (CHEMBL4225049) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461685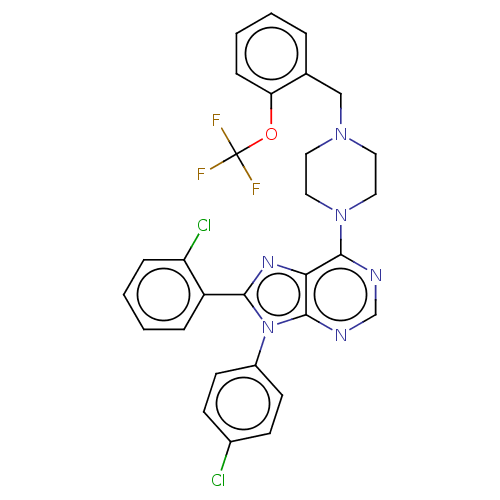 (CHEMBL4227442) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461693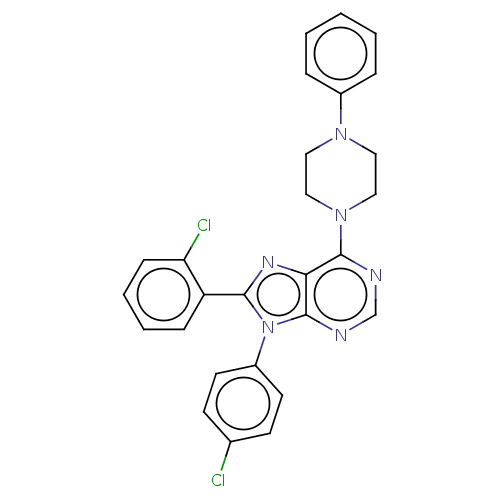 (CHEMBL4228096) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463423 (CHEMBL4249233) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 586 total ) | Next | Last >> |