 Found 427 hits with Last Name = 'tong' and Initial = 'w'
Found 427 hits with Last Name = 'tong' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50067497
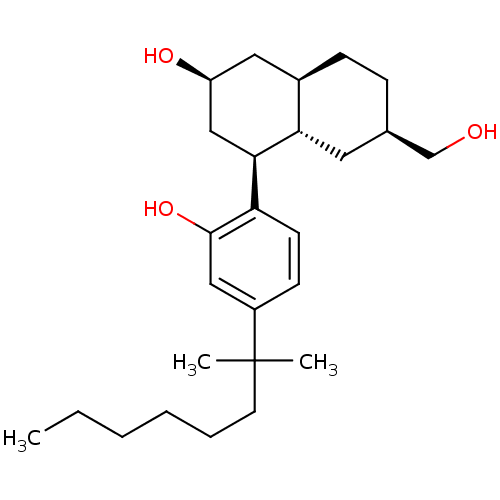
((2S,4S,4aS,6R,8aR)-4-[4-(1,1-Dimethyl-heptyl)-2-hy...)Show SMILES CCCCCCC(C)(C)c1ccc([C@H]2C[C@@H](O)C[C@H]3CC[C@@H](CO)C[C@H]23)c(O)c1 Show InChI InChI=1S/C26H42O3/c1-4-5-6-7-12-26(2,3)20-10-11-22(25(29)15-20)24-16-21(28)14-19-9-8-18(17-27)13-23(19)24/h10-11,15,18-19,21,23-24,27-29H,4-9,12-14,16-17H2,1-3H3/t18-,19-,21+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes |
J Med Chem 41: 4207-15 (1998)
Article DOI: 10.1021/jm970239z
BindingDB Entry DOI: 10.7270/Q2C24VK6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50472361
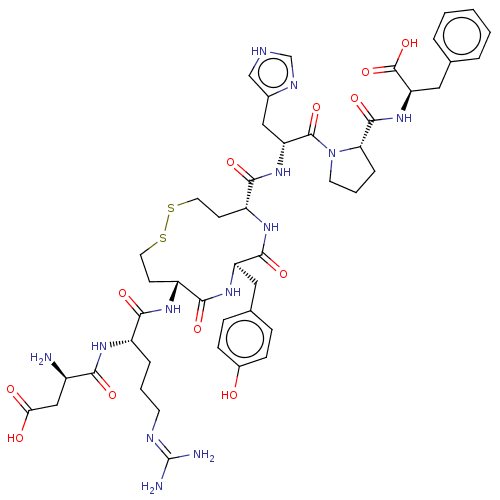
(CHEMBL406349)Show SMILES N[C@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CCSSCC[C@@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@H](Cc1ccccc1)C(O)=O |wU:59.63,46.47,9.8,31.31,wD:20.19,27.44,63.66,1.0,(-2.49,-6.28,;-2.47,-4.62,;-3.84,-3.93,;-5.11,-4.77,;-5.13,-6.21,;-6.53,-4.07,;-1.11,-3.93,;-1.11,-2.41,;.19,-4.74,;1.56,-4.01,;1.59,-2.47,;2.96,-1.73,;2.99,-.19,;4.35,.53,;4.39,2.07,;5.75,2.8,;3.09,2.89,;2.87,-4.81,;2.68,-3.3,;4.21,-5.54,;5.55,-4.77,;5.54,-3.23,;6.63,-2.13,;7.96,-1.36,;11.63,-.89,;13.17,-.89,;14.26,-1.99,;13.54,-3.36,;12.02,-3.43,;10.48,-3.48,;9.64,-2.18,;9.76,-4.85,;10.66,-6.02,;9.85,-7.26,;10.53,-8.56,;9.71,-9.78,;8.24,-9.71,;7.43,-10.95,;7.57,-8.41,;8.36,-7.17,;8.22,-4.77,;6.89,-5.54,;6.89,-7.08,;14.37,-4.67,;14.36,-6.19,;15.85,-4.25,;17.23,-4.78,;17.41,-6.19,;16.4,-7.33,;15.13,-6.82,;14.1,-7.96,;14.75,-9.19,;16.19,-8.82,;18.23,-3.64,;17.74,-2.18,;19.74,-3.95,;19.74,-2.41,;21.22,-1.94,;22.12,-3.13,;21.21,-4.39,;21.7,-5.86,;20.68,-7.01,;23.2,-6.16,;24.74,-6.17,;25.76,-4.98,;24.97,-3.64,;23.43,-3.64,;22.64,-2.29,;23.43,-.94,;24.97,-.94,;25.76,-2.29,;25.25,-7.63,;24.37,-8.87,;26.67,-7.65,)| Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31+,32-,33-,34-,35-,36-,37+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes |
J Med Chem 42: 4524-37 (1999)
Article DOI: 10.1021/jm991089q
BindingDB Entry DOI: 10.7270/Q2PN98C1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes |
J Med Chem 41: 4207-15 (1998)
Article DOI: 10.1021/jm970239z
BindingDB Entry DOI: 10.7270/Q2C24VK6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes |
J Med Chem 42: 4524-37 (1999)
Article DOI: 10.1021/jm991089q
BindingDB Entry DOI: 10.7270/Q2PN98C1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471981

(CHEMBL446629)Show SMILES Cn1cc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-37-23-31(25-10-4-2-5-11-25)36-32(37)20-21-41-27-18-16-24(17-19-27)22-30(34(39)40)35-29-15-9-8-14-28(29)33(38)26-12-6-3-7-13-26/h2-19,23,30,35H,20-22H2,1H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471983
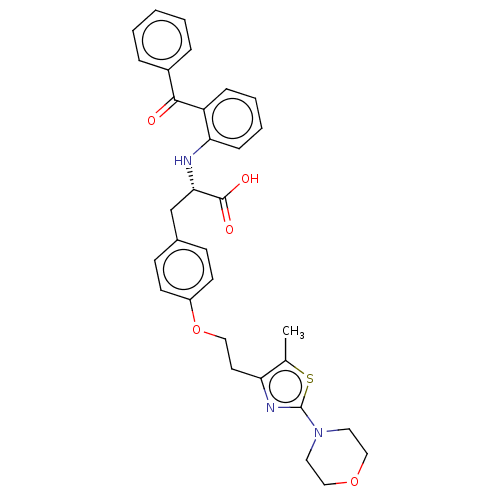
(CHEMBL149876)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCOCC1 Show InChI InChI=1S/C32H33N3O5S/c1-22-27(34-32(41-22)35-16-19-39-20-17-35)15-18-40-25-13-11-23(12-14-25)21-29(31(37)38)33-28-10-6-5-9-26(28)30(36)24-7-3-2-4-8-24/h2-14,29,33H,15-21H2,1H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471980
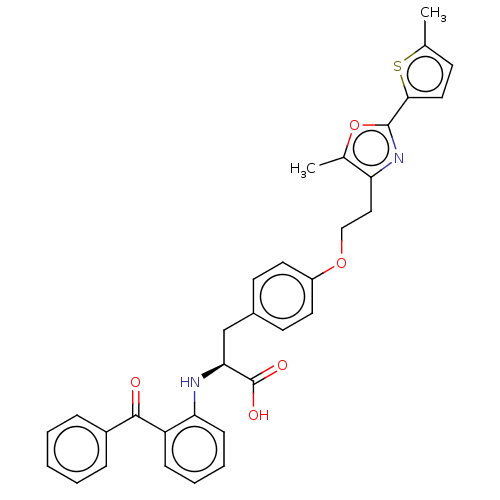
(CHEMBL147095)Show SMILES Cc1ccc(s1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C33H30N2O5S/c1-21-12-17-30(41-21)32-35-27(22(2)40-32)18-19-39-25-15-13-23(14-16-25)20-29(33(37)38)34-28-11-7-6-10-26(28)31(36)24-8-4-3-5-9-24/h3-17,29,34H,18-20H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471978

(CHEMBL147090)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccncc1 Show InChI InChI=1S/C33H29N3O4S/c1-22-28(36-32(41-22)25-15-18-34-19-16-25)17-20-40-26-13-11-23(12-14-26)21-30(33(38)39)35-29-10-6-5-9-27(29)31(37)24-7-3-2-4-8-24/h2-16,18-19,30,35H,17,20-21H2,1H3,(H,38,39)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471951

(CHEMBL343210)Show SMILES Cc1cc(no1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)s1 Show InChI InChI=1S/C32H29N3O5S/c1-20-18-28(35-40-20)31-34-26(21(2)41-31)16-17-39-24-14-12-22(13-15-24)19-29(32(37)38)33-27-11-7-6-10-25(27)30(36)23-8-4-3-5-9-23/h3-15,18,29,33H,16-17,19H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472023
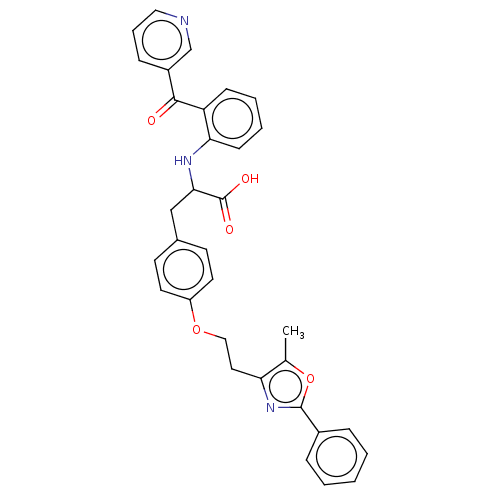
(CHEMBL148384)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccnc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H29N3O5/c1-22-28(36-32(41-22)24-8-3-2-4-9-24)17-19-40-26-15-13-23(14-16-26)20-30(33(38)39)35-29-12-6-5-11-27(29)31(37)25-10-7-18-34-21-25/h2-16,18,21,30,35H,17,19-20H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472019
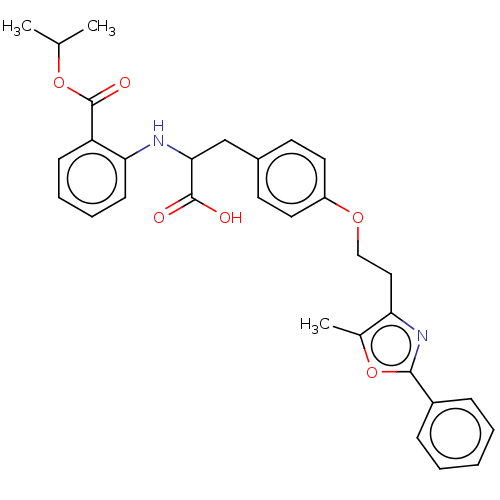
(CHEMBL148668)Show SMILES CC(C)OC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C31H32N2O6/c1-20(2)38-31(36)25-11-7-8-12-27(25)32-28(30(34)35)19-22-13-15-24(16-14-22)37-18-17-26-21(3)39-29(33-26)23-9-5-4-6-10-23/h4-16,20,28,32H,17-19H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471959
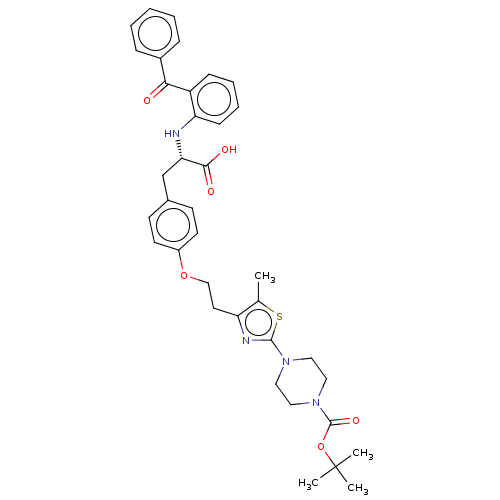
(CHEMBL146301)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCN(CC1)C(=O)OC(C)(C)C Show InChI InChI=1S/C37H42N4O6S/c1-25-30(39-35(48-25)40-19-21-41(22-20-40)36(45)47-37(2,3)4)18-23-46-28-16-14-26(15-17-28)24-32(34(43)44)38-31-13-9-8-12-29(31)33(42)27-10-6-5-7-11-27/h5-17,32,38H,18-24H2,1-4H3,(H,43,44)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471953
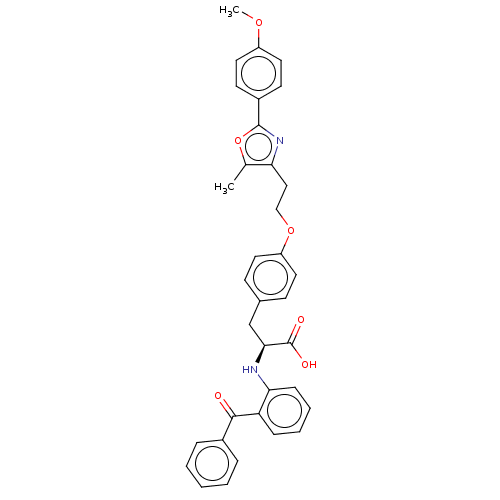
(CHEMBL356382)Show SMILES COc1ccc(cc1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C35H32N2O6/c1-23-30(37-34(43-23)26-14-18-27(41-2)19-15-26)20-21-42-28-16-12-24(13-17-28)22-32(35(39)40)36-31-11-7-6-10-29(31)33(38)25-8-4-3-5-9-25/h3-19,32,36H,20-22H2,1-2H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471968
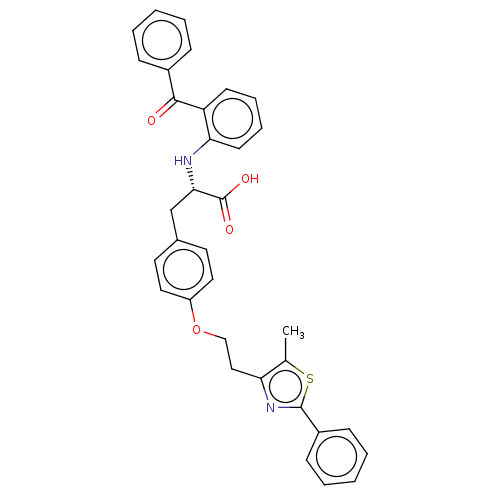
(CHEMBL358379)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O4S/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471966

(CHEMBL147935)Show SMILES Cc1cc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)nn1-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-24-22-27(36-37(24)28-12-6-3-7-13-28)20-21-41-29-18-16-25(17-19-29)23-32(34(39)40)35-31-15-9-8-14-30(31)33(38)26-10-4-2-5-11-26/h2-19,22,32,35H,20-21,23H2,1H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471974

(CHEMBL148950)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccncc1 Show InChI InChI=1S/C33H29N3O5/c1-22-28(36-32(41-22)25-15-18-34-19-16-25)17-20-40-26-13-11-23(12-14-26)21-30(33(38)39)35-29-10-6-5-9-27(29)31(37)24-7-3-2-4-8-24/h2-16,18-19,30,35H,17,20-21H2,1H3,(H,38,39)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472043
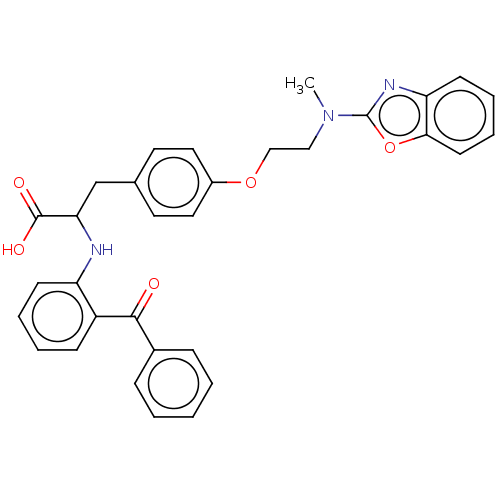
(CHEMBL148596)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472035
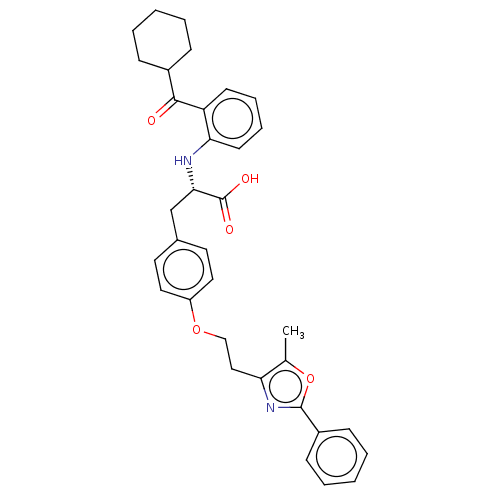
(CHEMBL2112870)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)C2CCCCC2)C(O)=O)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H36N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h3,6-9,12-19,25,31,35H,2,4-5,10-11,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471982
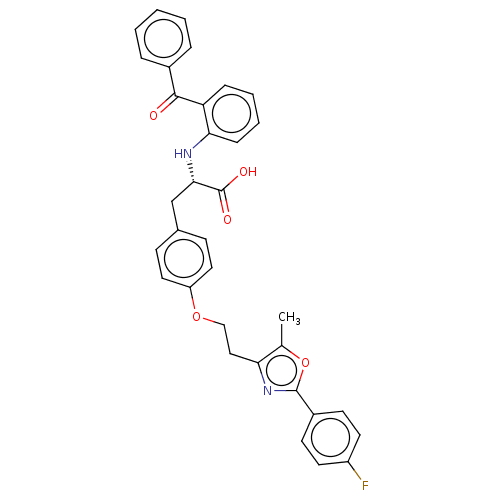
(CHEMBL147384)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H29FN2O5/c1-22-29(37-33(42-22)25-13-15-26(35)16-14-25)19-20-41-27-17-11-23(12-18-27)21-31(34(39)40)36-30-10-6-5-9-28(30)32(38)24-7-3-2-4-8-24/h2-18,31,36H,19-21H2,1H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472034
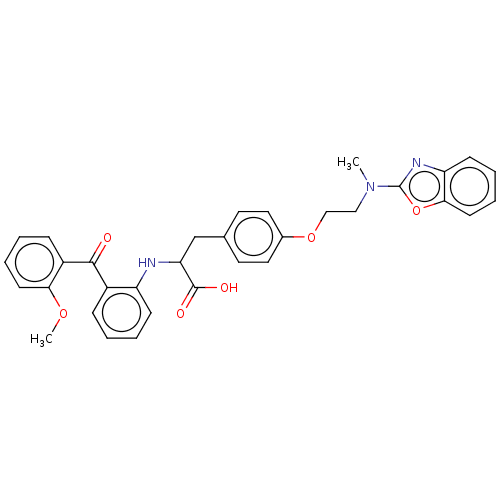
(CHEMBL147021)Show SMILES COc1ccccc1C(=O)c1ccccc1NC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-36(33-35-27-12-6-8-14-30(27)42-33)19-20-41-23-17-15-22(16-18-23)21-28(32(38)39)34-26-11-5-3-9-24(26)31(37)25-10-4-7-13-29(25)40-2/h3-18,28,34H,19-21H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472037

(CHEMBL414442)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccncc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H29N3O5/c1-22-28(36-32(41-22)25-7-3-2-4-8-25)17-20-40-26-13-11-23(12-14-26)21-30(33(38)39)35-29-10-6-5-9-27(29)31(37)24-15-18-34-19-16-24/h2-16,18-19,30,35H,17,20-21H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471955
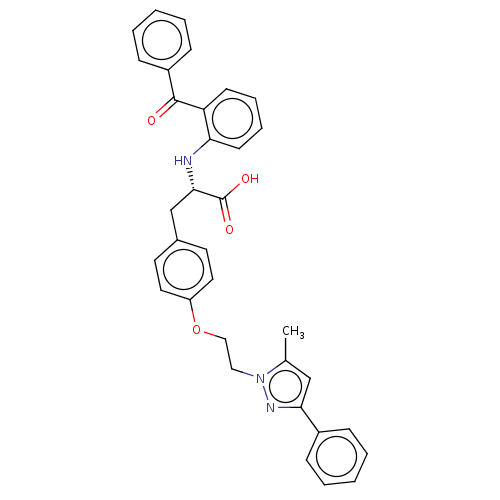
(CHEMBL148797)Show SMILES Cc1cc(nn1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-24-22-31(26-10-4-2-5-11-26)36-37(24)20-21-41-28-18-16-25(17-19-28)23-32(34(39)40)35-30-15-9-8-14-29(30)33(38)27-12-6-3-7-13-27/h2-19,22,32,35H,20-21,23H2,1H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471970

(CHEMBL148459)Show SMILES Cc1ccsc1-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C33H30N2O5S/c1-21-17-19-41-31(21)32-35-27(22(2)40-32)16-18-39-25-14-12-23(13-15-25)20-29(33(37)38)34-28-11-7-6-10-26(28)30(36)24-8-4-3-5-9-24/h3-15,17,19,29,34H,16,18,20H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471948
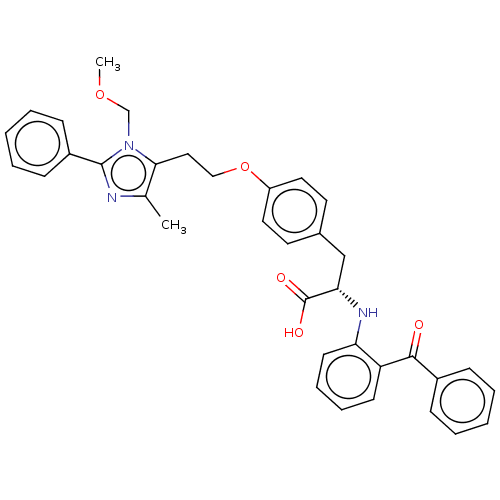
(CHEMBL149647)Show SMILES COCn1c(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)nc1-c1ccccc1 Show InChI InChI=1S/C36H35N3O5/c1-25-33(39(24-43-2)35(37-25)28-13-7-4-8-14-28)21-22-44-29-19-17-26(18-20-29)23-32(36(41)42)38-31-16-10-9-15-30(31)34(40)27-11-5-3-6-12-27/h3-20,32,38H,21-24H2,1-2H3,(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472047

(CHEMBL146355)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccccc2C)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-22-9-3-4-10-25(22)31(37)26-11-5-6-12-27(26)34-29(32(38)39)21-23-15-17-24(18-16-23)40-20-19-36(2)33-35-28-13-7-8-14-30(28)41-33/h3-18,29,34H,19-21H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471954
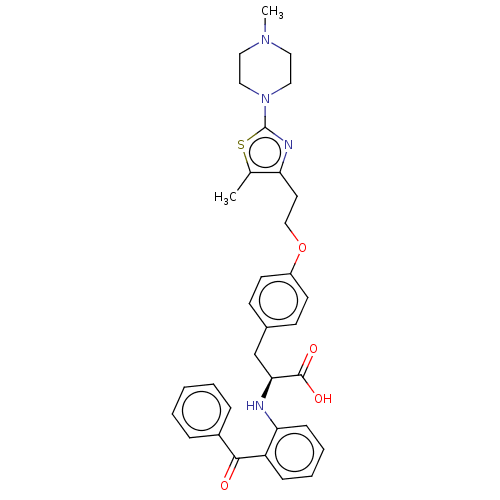
(CHEMBL358325)Show SMILES CN1CCN(CC1)c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)s1 Show InChI InChI=1S/C33H36N4O4S/c1-23-28(35-33(42-23)37-19-17-36(2)18-20-37)16-21-41-26-14-12-24(13-15-26)22-30(32(39)40)34-29-11-7-6-10-27(29)31(38)25-8-4-3-5-9-25/h3-15,30,34H,16-22H2,1-2H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472048

(CHEMBL146562)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccsc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C30H27N3O5S/c1-33(30-32-25-8-4-5-9-27(25)38-30)15-16-37-22-12-10-20(11-13-22)18-26(29(35)36)31-24-7-3-2-6-23(24)28(34)21-14-17-39-19-21/h2-14,17,19,26,31H,15-16,18H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472024

(CHEMBL145886)Show SMILES COc1cccc(c1)C(=O)c1ccccc1NC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-36(33-35-28-12-5-6-13-30(28)42-33)18-19-41-24-16-14-22(15-17-24)20-29(32(38)39)34-27-11-4-3-10-26(27)31(37)23-8-7-9-25(21-23)40-2/h3-17,21,29,34H,18-20H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472041

(CHEMBL147770)Show SMILES CCCOC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C31H32N2O6/c1-3-18-38-31(36)25-11-7-8-12-27(25)32-28(30(34)35)20-22-13-15-24(16-14-22)37-19-17-26-21(2)39-29(33-26)23-9-5-4-6-10-23/h4-16,28,32H,3,17-20H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471991
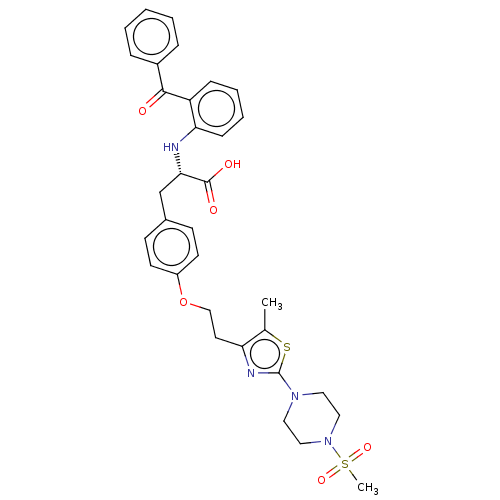
(CHEMBL146650)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C33H36N4O6S2/c1-23-28(35-33(44-23)36-17-19-37(20-18-36)45(2,41)42)16-21-43-26-14-12-24(13-15-26)22-30(32(39)40)34-29-11-7-6-10-27(29)31(38)25-8-4-3-5-9-25/h3-15,30,34H,16-22H2,1-2H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471989

(CHEMBL149835)Show SMILES Cc1[nH]c(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O4/c1-23-29(37-33(35-23)26-12-6-3-7-13-26)20-21-41-27-18-16-24(17-19-27)22-31(34(39)40)36-30-15-9-8-14-28(30)32(38)25-10-4-2-5-11-25/h2-19,31,36H,20-22H2,1H3,(H,35,37)(H,39,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472029

(CHEMBL149780)Show SMILES CN(CCOc1ccc(CC(Nc2cscc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C30H27N3O5S/c1-33(30-32-24-9-5-6-10-27(24)38-30)15-16-37-22-13-11-20(12-14-22)17-25(29(35)36)31-26-19-39-18-23(26)28(34)21-7-3-2-4-8-21/h2-14,18-19,25,31H,15-17H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472021

(CHEMBL146637)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccc(c2)C(F)(F)F)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H28F3N3O5/c1-39(32-38-27-11-4-5-12-29(27)44-32)17-18-43-24-15-13-21(14-16-24)19-28(31(41)42)37-26-10-3-2-9-25(26)30(40)22-7-6-8-23(20-22)33(34,35)36/h2-16,20,28,37H,17-19H2,1H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471963

(CHEMBL359285)Show SMILES COCCNc1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)s1 Show InChI InChI=1S/C31H33N3O5S/c1-21-26(34-31(40-21)32-17-19-38-2)16-18-39-24-14-12-22(13-15-24)20-28(30(36)37)33-27-11-7-6-10-25(27)29(35)23-8-4-3-5-9-23/h3-15,28,33H,16-20H2,1-2H3,(H,32,34)(H,36,37)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472031

(CHEMBL146579)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccccc2C(F)(F)F)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H28F3N3O5/c1-39(32-38-27-12-6-7-13-29(27)44-32)18-19-43-22-16-14-21(15-17-22)20-28(31(41)42)37-26-11-5-3-9-24(26)30(40)23-8-2-4-10-25(23)33(34,35)36/h2-17,28,37H,18-20H2,1H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472028

(CHEMBL150215)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccc(C)c2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-22-8-7-9-24(20-22)31(37)26-10-3-4-11-27(26)34-29(32(38)39)21-23-14-16-25(17-15-23)40-19-18-36(2)33-35-28-12-5-6-13-30(28)41-33/h3-17,20,29,34H,18-19,21H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472025

(CHEMBL150232)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccc(C)cc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-22-11-15-24(16-12-22)31(37)26-7-3-4-8-27(26)34-29(32(38)39)21-23-13-17-25(18-14-23)40-20-19-36(2)33-35-28-9-5-6-10-30(28)41-33/h3-18,29,34H,19-21H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472032
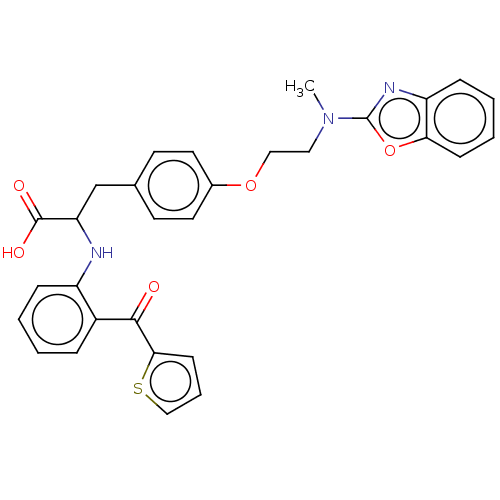
(CHEMBL146584)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccs2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C30H27N3O5S/c1-33(30-32-24-9-4-5-10-26(24)38-30)16-17-37-21-14-12-20(13-15-21)19-25(29(35)36)31-23-8-3-2-7-22(23)28(34)27-11-6-18-39-27/h2-15,18,25,31H,16-17,19H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472011

(CHEMBL148300)Show SMILES CCOC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C30H30N2O6/c1-3-36-30(35)24-11-7-8-12-26(24)31-27(29(33)34)19-21-13-15-23(16-14-21)37-18-17-25-20(2)38-28(32-25)22-9-5-4-6-10-22/h4-16,27,31H,3,17-19H2,1-2H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472052

(CHEMBL145958)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccc3ccccc23)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C36H31N3O5/c1-39(36-38-31-15-6-7-16-33(31)44-36)21-22-43-26-19-17-24(18-20-26)23-32(35(41)42)37-30-14-5-4-12-29(30)34(40)28-13-8-10-25-9-2-3-11-27(25)28/h2-20,32,37H,21-23H2,1H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibiting the 50% binding of Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472033
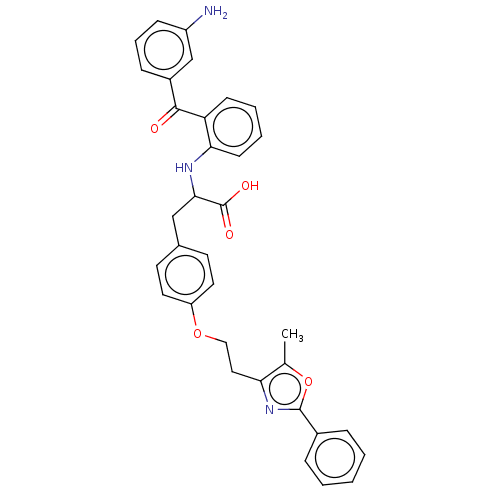
(CHEMBL347788)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccc(N)c2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H31N3O5/c1-22-29(37-33(42-22)24-8-3-2-4-9-24)18-19-41-27-16-14-23(15-17-27)20-31(34(39)40)36-30-13-6-5-12-28(30)32(38)25-10-7-11-26(35)21-25/h2-17,21,31,36H,18-20,35H2,1H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472014
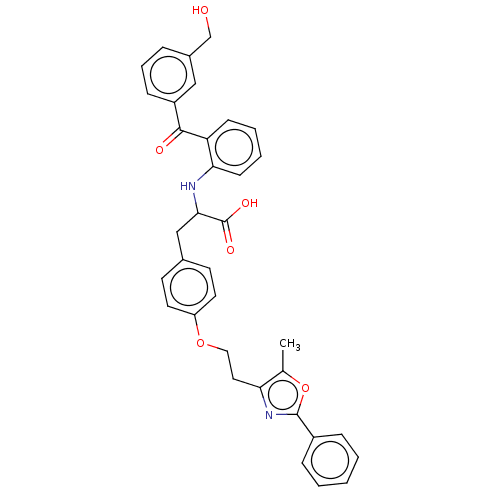
(CHEMBL146530)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2cccc(CO)c2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C35H32N2O6/c1-23-30(37-34(43-23)26-9-3-2-4-10-26)18-19-42-28-16-14-24(15-17-28)21-32(35(40)41)36-31-13-6-5-12-29(31)33(39)27-11-7-8-25(20-27)22-38/h2-17,20,32,36,38H,18-19,21-22H2,1H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085046
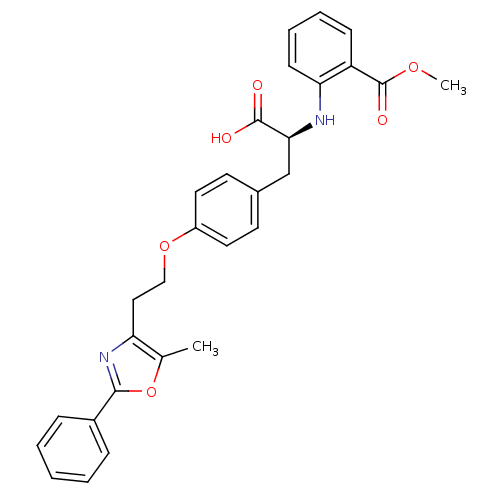
(2-((S)-1-carboxy-2-{4-[2-(5-methyl-2-phenyl-oxazol...)Show SMILES COC(=O)c1ccccc1N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C29H28N2O6/c1-19-24(31-27(37-19)21-8-4-3-5-9-21)16-17-36-22-14-12-20(13-15-22)18-26(28(32)33)30-25-11-7-6-10-23(25)29(34)35-2/h3-15,26,30H,16-18H2,1-2H3,(H,32,33)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472049
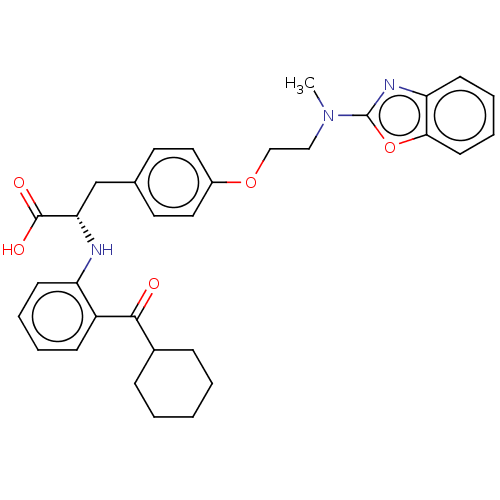
(CHEMBL2112871)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)C2CCCCC2)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C32H35N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h5-8,11-18,23,28,33H,2-4,9-10,19-21H2,1H3,(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472044
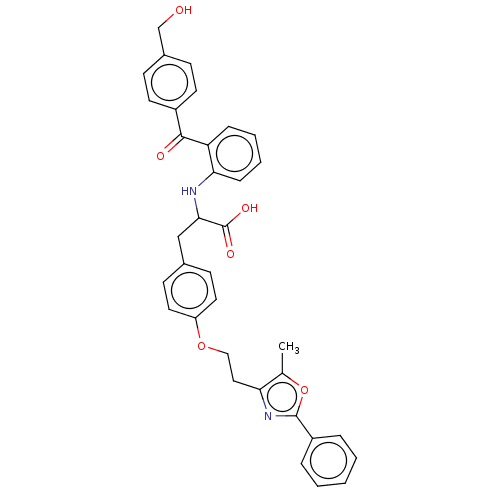
(CHEMBL145824)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccc(CO)cc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C35H32N2O6/c1-23-30(37-34(43-23)27-7-3-2-4-8-27)19-20-42-28-17-13-24(14-18-28)21-32(35(40)41)36-31-10-6-5-9-29(31)33(39)26-15-11-25(22-38)12-16-26/h2-18,32,36,38H,19-22H2,1H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471973
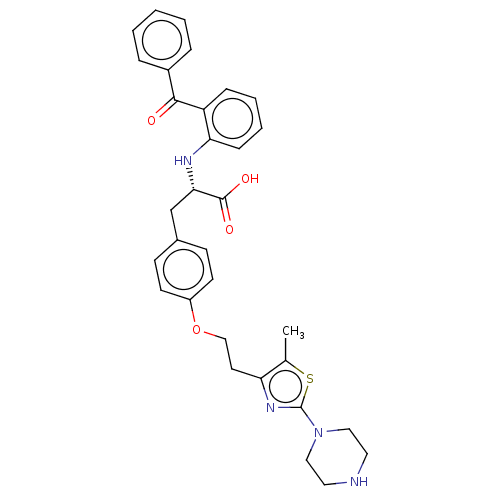
(CHEMBL146231)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)N1CCNCC1 Show InChI InChI=1S/C32H34N4O4S/c1-22-27(35-32(41-22)36-18-16-33-17-19-36)15-20-40-25-13-11-23(12-14-25)21-29(31(38)39)34-28-10-6-5-9-26(28)30(37)24-7-3-2-4-8-24/h2-14,29,33-34H,15-21H2,1H3,(H,38,39)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471967
(CHEMBL342292)Show SMILES Cc1nc(nn1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H30N4O4/c1-23-34-32(26-12-6-3-7-13-26)36-37(23)20-21-41-27-18-16-24(17-19-27)22-30(33(39)40)35-29-15-9-8-14-28(29)31(38)25-10-4-2-5-11-25/h2-19,30,35H,20-22H2,1H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472018
(CHEMBL146217)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)c2ccc(cc2)C(F)(F)F)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H28F3N3O5/c1-39(32-38-27-8-4-5-9-29(27)44-32)18-19-43-24-16-10-21(11-17-24)20-28(31(41)42)37-26-7-3-2-6-25(26)30(40)22-12-14-23(15-13-22)33(34,35)36/h2-17,28,37H,18-20H2,1H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma) |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data