 Found 62 hits with Last Name = 'wilson' and Initial = 'wd'
Found 62 hits with Last Name = 'wilson' and Initial = 'wd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470684

(CHEMBL173975)Show SMILES Cl.Cl.C1CNC(=NC1)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NCCCN1 |c:3,t:27| Show InChI InChI=1S/C24H24N4O/c1-13-25-23(26-14-1)19-7-3-17(4-8-19)21-11-12-22(29-21)18-5-9-20(10-6-18)24-27-15-2-16-28-24/h3-12H,1-2,13-16H2,(H,25,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470690
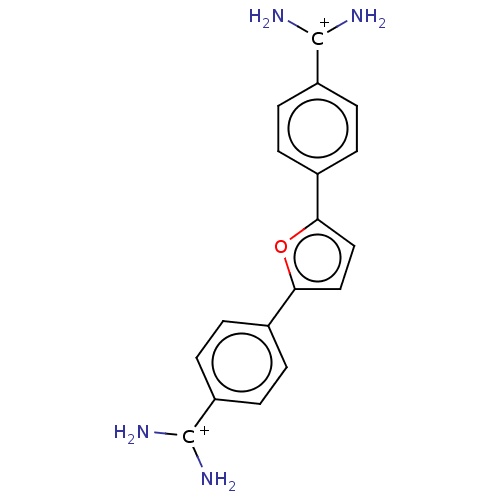
(CHEMBL367883)Show SMILES [Cl-].[Cl-].N[C+](N)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)[C+](N)N Show InChI InChI=1S/C18H16N4O/c19-17(20)13-5-1-11(2-6-13)15-9-10-16(23-15)12-3-7-14(8-4-12)18(21)22/h1-10H,(H3,19,20)(H3,21,22)/p+2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470688

(CHEMBL170751)Show SMILES Cl.Cl.C1CCN=C(NC1)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NCCCCN1 |c:3,t:28| Show InChI InChI=1S/C26H28N4O/c1-2-16-28-25(27-15-1)21-9-5-19(6-10-21)23-13-14-24(31-23)20-7-11-22(12-8-20)26-29-17-3-4-18-30-26/h5-14H,1-4,15-18H2,(H,27,28)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470689
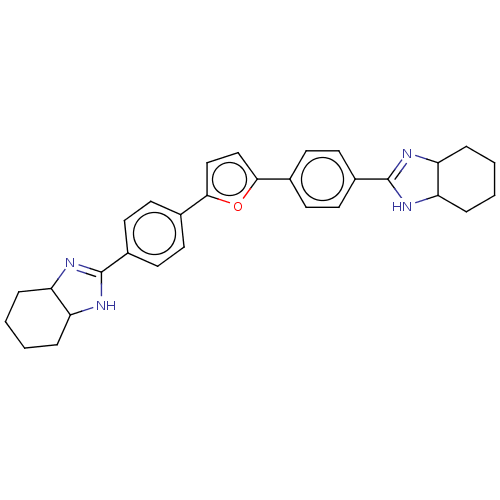
(CHEMBL171287)Show SMILES Cl.Cl.C1CCC2N=C(NC2C1)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NC2CCCCC2N1 |c:4,t:31| Show InChI InChI=1S/C30H32N4O.2ClH/c1-2-6-24-23(5-1)31-29(32-24)21-13-9-19(10-14-21)27-17-18-28(35-27)20-11-15-22(16-12-20)30-33-25-7-3-4-8-26(25)34-30;;/h9-18,23-26H,1-8H2,(H,31,32)(H,33,34);2*1H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470685
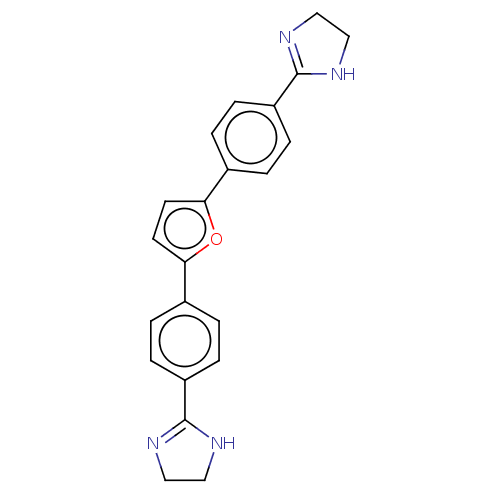
(CHEMBL557158)Show SMILES Cl.C1CN=C(N1)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NCCN1 |c:2,t:26| Show InChI InChI=1S/C22H20N4O/c1-5-17(21-23-11-12-24-21)6-2-15(1)19-9-10-20(27-19)16-3-7-18(8-4-16)22-25-13-14-26-22/h1-10H,11-14H2,(H,23,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470686
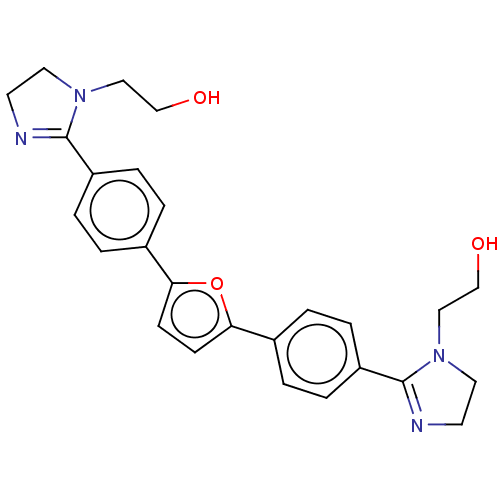
(CHEMBL170447)Show SMILES Cl.Cl.OCCN1CCN=C1c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NCCN1CCO |c:6,t:29| Show InChI InChI=1S/C26H28N4O3/c31-17-15-29-13-11-27-25(29)21-5-1-19(2-6-21)23-9-10-24(33-23)20-3-7-22(8-4-20)26-28-12-14-30(26)16-18-32/h1-10,31-32H,11-18H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470687
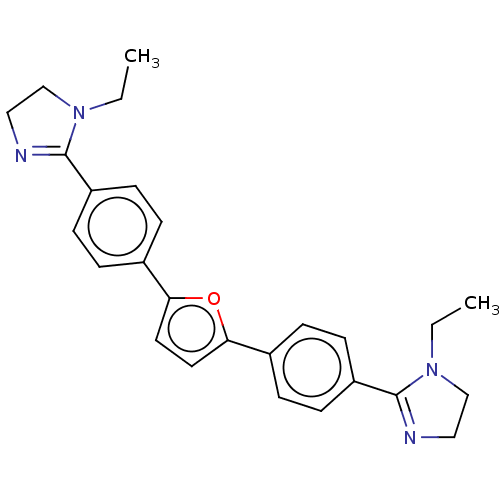
(CHEMBL171707)Show SMILES Cl.Cl.CCN1CCN=C1c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NCCN1CC |c:5,t:28| Show InChI InChI=1S/C26H28N4O/c1-3-29-17-15-27-25(29)21-9-5-19(6-10-21)23-13-14-24(31-23)20-7-11-22(12-8-20)26-28-16-18-30(26)4-2/h5-14H,3-4,15-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134014

(CHEMBL343041 | N,N'-(9-(2-hydroxyphenylamino)acrid...)Show SMILES Oc1ccccc1Nc1c2cc(NC(=O)CCN3CCCC3)ccc2nc2ccc(NC(=O)CCN3CCCC3)cc12 Show InChI InChI=1S/C33H38N6O3/c40-30-8-2-1-7-29(30)37-33-25-21-23(34-31(41)13-19-38-15-3-4-16-38)9-11-27(25)36-28-12-10-24(22-26(28)33)35-32(42)14-20-39-17-5-6-18-39/h1-2,7-12,21-22,40H,3-6,13-20H2,(H,34,41)(H,35,42)(H,36,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134018
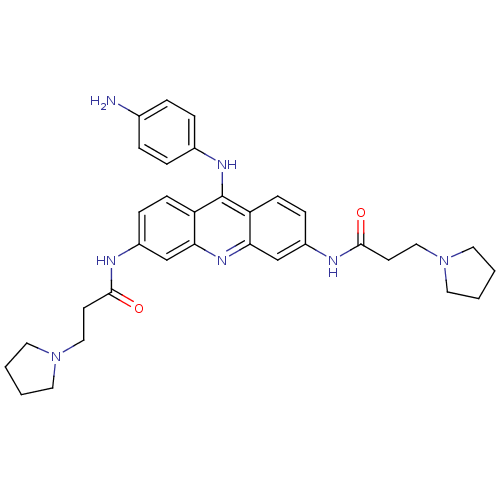
(CHEMBL335819 | N,N'-(9-(4-aminophenylamino)acridin...)Show SMILES Nc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C33H39N7O2/c34-23-5-7-24(8-6-23)37-33-27-11-9-25(35-31(41)13-19-39-15-1-2-16-39)21-29(27)38-30-22-26(10-12-28(30)33)36-32(42)14-20-40-17-3-4-18-40/h5-12,21-22H,1-4,13-20,34H2,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50133998

(CHEMBL137809 | N,N'-(9-(2-(methylthio)phenylamino)...)Show SMILES CSc1ccccc1Nc1c2ccc(NC(=O)CCN3CCCC3)cc2nc2cc(NC(=O)CCN3CCCC3)ccc12 Show InChI InChI=1S/C34H40N6O2S/c1-43-31-9-3-2-8-28(31)38-34-26-12-10-24(35-32(41)14-20-39-16-4-5-17-39)22-29(26)37-30-23-25(11-13-27(30)34)36-33(42)15-21-40-18-6-7-19-40/h2-3,8-13,22-23H,4-7,14-21H2,1H3,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134024

(CHEMBL139500 | N,N'-(9-(3-aminophenylamino)acridin...)Show SMILES Nc1cccc(Nc2c3cc(NC(=O)CCN4CCCC4)ccc3nc3ccc(NC(=O)CCN4CCCC4)cc23)c1 Show InChI InChI=1S/C33H39N7O2/c34-23-6-5-7-24(20-23)37-33-27-21-25(35-31(41)12-18-39-14-1-2-15-39)8-10-29(27)38-30-11-9-26(22-28(30)33)36-32(42)13-19-40-16-3-4-17-40/h5-11,20-22H,1-4,12-19,34H2,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080849
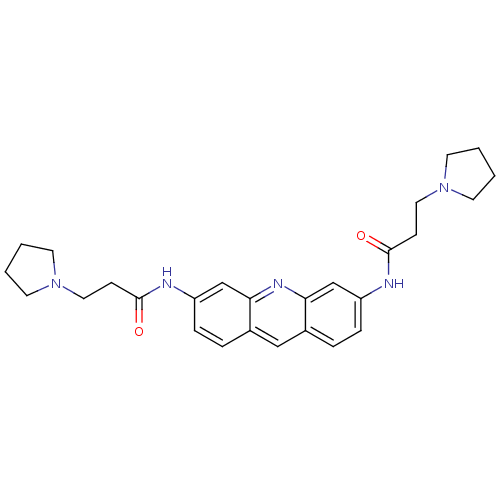
(3,6-Bis(3-pyrrolidinopropionamido)acridine | 3-PYR...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C27H33N5O2/c33-26(9-15-31-11-1-2-12-31)28-22-7-5-20-17-21-6-8-23(19-25(21)30-24(20)18-22)29-27(34)10-16-32-13-3-4-14-32/h5-8,17-19H,1-4,9-16H2,(H,28,33)(H,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134025

(CHEMBL343795 | N,N'-(9-(4-fluorophenylamino)acridi...)Show SMILES Fc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C33H37FN6O2/c34-23-5-7-24(8-6-23)37-33-27-11-9-25(35-31(41)13-19-39-15-1-2-16-39)21-29(27)38-30-22-26(10-12-28(30)33)36-32(42)14-20-40-17-3-4-18-40/h5-12,21-22H,1-4,13-20H2,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134021
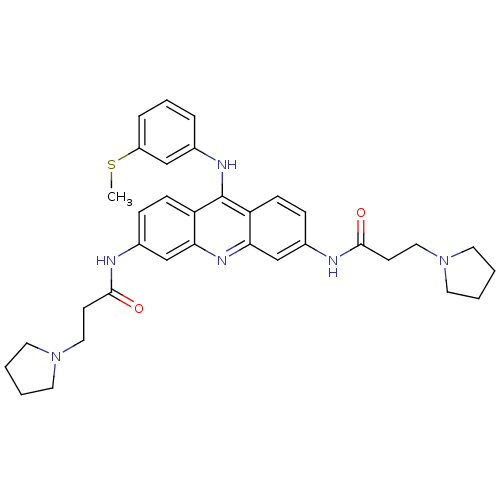
(CHEMBL138761 | N,N'-(9-(3-(methylthio)phenylamino)...)Show SMILES CSc1cccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)c1 Show InChI InChI=1S/C34H40N6O2S/c1-43-27-8-6-7-24(21-27)37-34-28-11-9-25(35-32(41)13-19-39-15-2-3-16-39)22-30(28)38-31-23-26(10-12-29(31)34)36-33(42)14-20-40-17-4-5-18-40/h6-12,21-23H,2-5,13-20H2,1H3,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134029
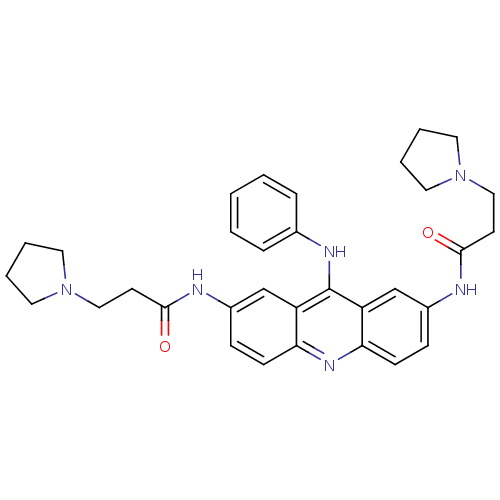
(CHEMBL138369 | N,N'-(9-(phenylamino)acridine-2,7-d...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2nc3ccc(NC(=O)CCN4CCCC4)cc3c(Nc3ccccc3)c2c1 Show InChI InChI=1S/C33H38N6O2/c40-31(14-20-38-16-4-5-17-38)34-25-10-12-29-27(22-25)33(36-24-8-2-1-3-9-24)28-23-26(11-13-30(28)37-29)35-32(41)15-21-39-18-6-7-19-39/h1-3,8-13,22-23H,4-7,14-21H2,(H,34,40)(H,35,41)(H,36,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134016
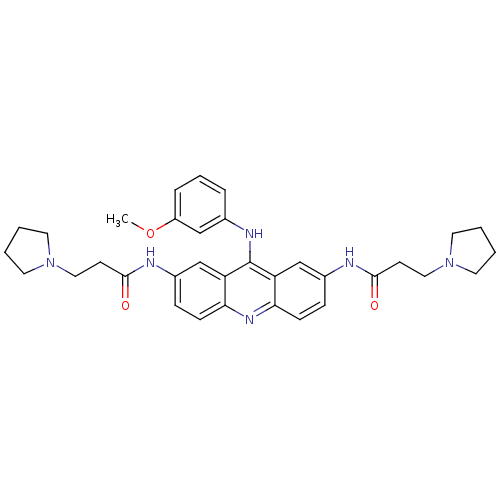
(CHEMBL342921 | N,N'-(9-(3-methoxyphenylamino)acrid...)Show SMILES COc1cccc(Nc2c3cc(NC(=O)CCN4CCCC4)ccc3nc3ccc(NC(=O)CCN4CCCC4)cc23)c1 Show InChI InChI=1S/C34H40N6O3/c1-43-27-8-6-7-24(21-27)37-34-28-22-25(35-32(41)13-19-39-15-2-3-16-39)9-11-30(28)38-31-12-10-26(23-29(31)34)36-33(42)14-20-40-17-4-5-18-40/h6-12,21-23H,2-5,13-20H2,1H3,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134002
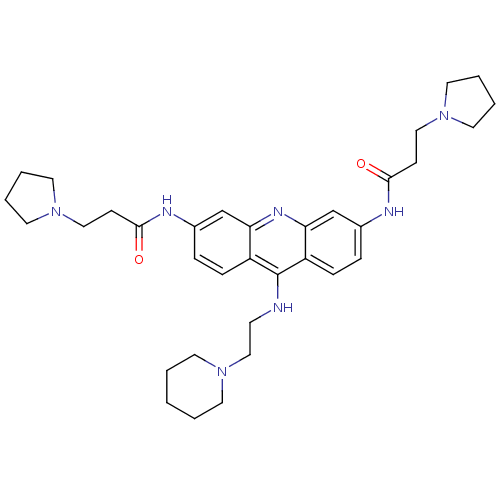
(CHEMBL141540 | N,N'-(9-(2-(piperidin-1-yl)ethylami...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2c(NCCN3CCCCC3)c3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C34H47N7O2/c42-32(12-21-39-17-4-5-18-39)36-26-8-10-28-30(24-26)38-31-25-27(37-33(43)13-22-40-19-6-7-20-40)9-11-29(31)34(28)35-14-23-41-15-2-1-3-16-41/h8-11,24-25H,1-7,12-23H2,(H,35,38)(H,36,42)(H,37,43) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134028

(CHEMBL142036 | N,N'-(9-(4-aminophenylamino)acridin...)Show SMILES Nc1ccc(Nc2c3cc(NC(=O)CCN4CCCC4)ccc3nc3ccc(NC(=O)CCN4CCCC4)cc23)cc1 Show InChI InChI=1S/C33H39N7O2/c34-23-5-7-24(8-6-23)37-33-27-21-25(35-31(41)13-19-39-15-1-2-16-39)9-11-29(27)38-30-12-10-26(22-28(30)33)36-32(42)14-20-40-17-3-4-18-40/h5-12,21-22H,1-4,13-20,34H2,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134019

(CHEMBL336417 | N,N'-(9-(3-aminophenylamino)acridin...)Show SMILES Nc1cccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)c1 Show InChI InChI=1S/C33H39N7O2/c34-23-6-5-7-24(20-23)37-33-27-10-8-25(35-31(41)12-18-39-14-1-2-15-39)21-29(27)38-30-22-26(9-11-28(30)33)36-32(42)13-19-40-16-3-4-17-40/h5-11,20-22H,1-4,12-19,34H2,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134032

(CHEMBL138811 | N,N'-(9-(cyclopropylamino)acridine-...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2c(NC3CC3)c3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C30H38N6O2/c37-28(11-17-35-13-1-2-14-35)31-22-7-9-24-26(19-22)34-27-20-23(8-10-25(27)30(24)33-21-5-6-21)32-29(38)12-18-36-15-3-4-16-36/h7-10,19-21H,1-6,11-18H2,(H,31,37)(H,32,38)(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134030
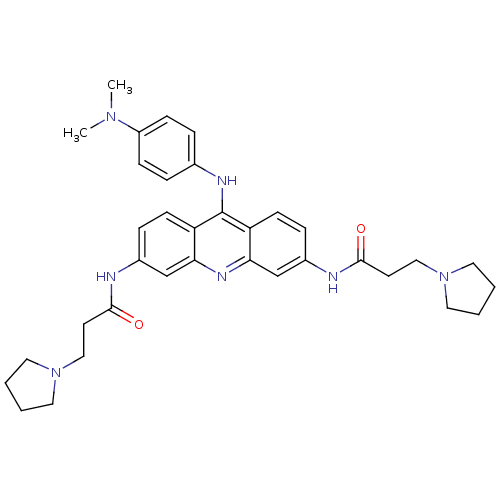
(9-[4-(N,N-dimethylamino)phenylamino]-3,6-bis(3-pyr...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-13-9-26(36-33(43)15-21-41-17-3-4-18-41)23-31(29)39-32-24-27(10-14-30(32)35)37-34(44)16-22-42-19-5-6-20-42/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134031

(CHEMBL137973 | N,N'-(9-(cyclohexylamino)acridine-2...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2c(NC3CCCCC3)c3cc(NC(=O)CCN4CCCC4)ccc3nc2c1 Show InChI InChI=1S/C33H44N6O2/c40-31(14-20-38-16-4-5-17-38)34-25-11-13-29-28(22-25)33(36-24-8-2-1-3-9-24)27-12-10-26(23-30(27)37-29)35-32(41)15-21-39-18-6-7-19-39/h10-13,22-24H,1-9,14-21H2,(H,34,40)(H,35,41)(H,36,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134013
(CHEMBL341985 | N,N'-(9-(4-(dimethylamino)phenylami...)Show SMILES CN(C)c1ccc(Nc2c3cc(NC(=O)CCN4CCCC4)ccc3nc3ccc(NC(=O)CCN4CCCC4)cc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-23-26(36-33(43)15-21-41-17-3-4-18-41)9-13-31(29)39-32-14-10-27(24-30(32)35)37-34(44)16-22-42-19-5-6-20-42/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134022
(CHEMBL336444 | N,N'-(9-(4-acetylphenylamino)acridi...)Show SMILES CC(=O)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C35H40N6O3/c1-24(42)25-6-8-26(9-7-25)38-35-29-12-10-27(36-33(43)14-20-40-16-2-3-17-40)22-31(29)39-32-23-28(11-13-30(32)35)37-34(44)15-21-41-18-4-5-19-41/h6-13,22-23H,2-5,14-21H2,1H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134017
(CHEMBL138357 | N,N'-(9-(4-(dimethylamino)phenylami...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3ccc(NC(=O)CCN4CCCC4)cc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-13-9-27(37-34(44)16-22-42-19-5-6-20-42)24-32(29)39-31-14-10-26(23-30(31)35)36-33(43)15-21-41-17-3-4-18-41/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134026
(CHEMBL138487 | N,N'-(9-(3-(dimethylamino)phenylami...)Show SMILES CN(C)c1cccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)c1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-9-7-8-25(22-28)38-35-29-12-10-26(36-33(43)14-20-41-16-3-4-17-41)23-31(29)39-32-24-27(11-13-30(32)35)37-34(44)15-21-42-18-5-6-19-42/h7-13,22-24H,3-6,14-21H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134033
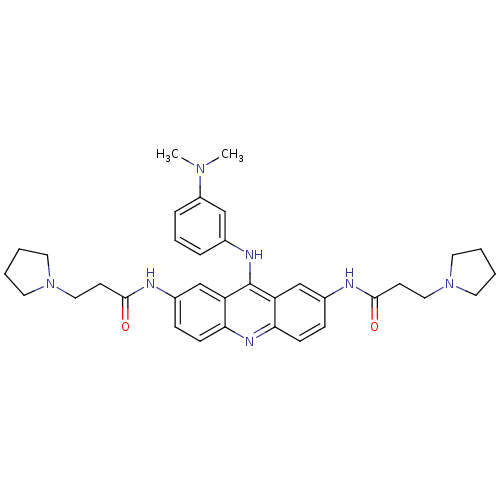
(CHEMBL139443 | N,N'-(9-(3-(dimethylamino)phenylami...)Show SMILES CN(C)c1cccc(Nc2c3cc(NC(=O)CCN4CCCC4)ccc3nc3ccc(NC(=O)CCN4CCCC4)cc23)c1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-9-7-8-25(22-28)38-35-29-23-26(36-33(43)14-20-41-16-3-4-17-41)10-12-31(29)39-32-13-11-27(24-30(32)35)37-34(44)15-21-42-18-5-6-19-42/h7-13,22-24H,3-6,14-21H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134015
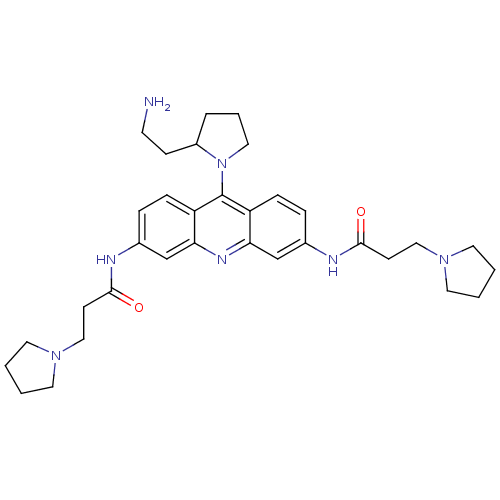
(CHEMBL44130 | N-[9-[2-(2-Amino-ethyl)-pyrrolidin-1...)Show SMILES NCCC1CCCN1c1c2ccc(NC(=O)CCN3CCCC3)cc2nc2cc(NC(=O)CCN3CCCC3)ccc12 Show InChI InChI=1S/C33H45N7O2/c34-14-11-26-6-5-19-40(26)33-27-9-7-24(35-31(41)12-20-38-15-1-2-16-38)22-29(27)37-30-23-25(8-10-28(30)33)36-32(42)13-21-39-17-3-4-18-39/h7-10,22-23,26H,1-6,11-21,34H2,(H,35,41)(H,36,42) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134008
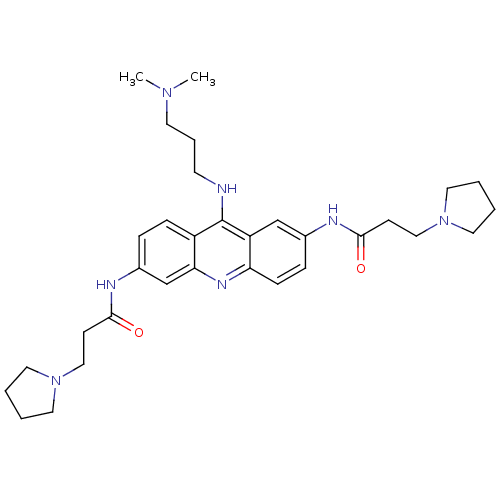
(CHEMBL138187 | N,N'-(9-(3-(dimethylamino)propylami...)Show SMILES CN(C)CCCNc1c2ccc(NC(=O)CCN3CCCC3)cc2nc2ccc(NC(=O)CCN3CCCC3)cc12 Show InChI InChI=1S/C32H45N7O2/c1-37(2)15-7-14-33-32-26-10-8-25(35-31(41)13-21-39-18-5-6-19-39)23-29(26)36-28-11-9-24(22-27(28)32)34-30(40)12-20-38-16-3-4-17-38/h8-11,22-23H,3-7,12-21H2,1-2H3,(H,33,36)(H,34,40)(H,35,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134023

(CHEMBL337762 | N,N'-(9-(4-aminophenylamino)acridin...)Show SMILES Nc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3ccc(NC(=O)CCN4CCCC4)cc23)cc1 Show InChI InChI=1S/C33H39N7O2/c34-23-5-7-24(8-6-23)37-33-27-11-9-26(36-32(42)14-20-40-17-3-4-18-40)22-30(27)38-29-12-10-25(21-28(29)33)35-31(41)13-19-39-15-1-2-16-39/h5-12,21-22H,1-4,13-20,34H2,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134006

(CHEMBL335564 | N,N'-(9-(2-methoxyethylamino)acridi...)Show SMILES COCCNc1c2ccc(NC(=O)CCN3CCCC3)cc2nc2cc(NC(=O)CCN3CCCC3)ccc12 Show InChI InChI=1S/C30H40N6O3/c1-39-19-12-31-30-24-8-6-22(32-28(37)10-17-35-13-2-3-14-35)20-26(24)34-27-21-23(7-9-25(27)30)33-29(38)11-18-36-15-4-5-16-36/h6-9,20-21H,2-5,10-19H2,1H3,(H,31,34)(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134027
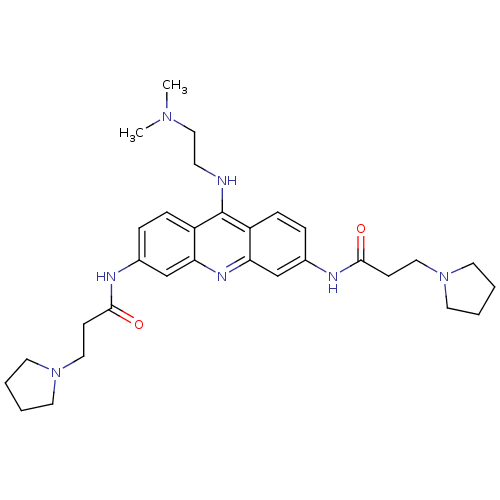
(CHEMBL140180 | N,N'-(9-(2-(dimethylamino)ethylamin...)Show SMILES CN(C)CCNc1c2ccc(NC(=O)CCN3CCCC3)cc2nc2cc(NC(=O)CCN3CCCC3)ccc12 Show InChI InChI=1S/C31H43N7O2/c1-36(2)20-13-32-31-25-9-7-23(33-29(39)11-18-37-14-3-4-15-37)21-27(25)35-28-22-24(8-10-26(28)31)34-30(40)12-19-38-16-5-6-17-38/h7-10,21-22H,3-6,11-20H2,1-2H3,(H,32,35)(H,33,39)(H,34,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134020
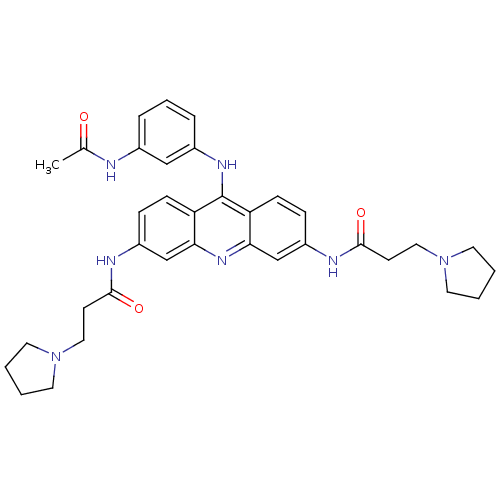
(CHEMBL141661 | N,N'-(9-(3-acetamidophenylamino)acr...)Show SMILES CC(=O)Nc1cccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)c1 Show InChI InChI=1S/C35H41N7O3/c1-24(43)36-25-7-6-8-26(21-25)39-35-29-11-9-27(37-33(44)13-19-41-15-2-3-16-41)22-31(29)40-32-23-28(10-12-30(32)35)38-34(45)14-20-42-17-4-5-18-42/h6-12,21-23H,2-5,13-20H2,1H3,(H,36,43)(H,37,44)(H,38,45)(H,39,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134003
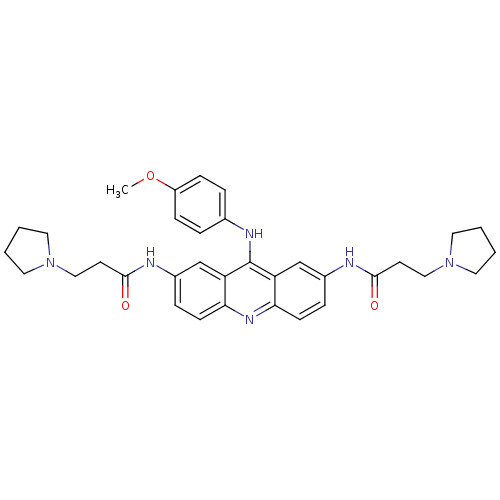
(CHEMBL141740 | N,N'-(9-(4-methoxyphenylamino)acrid...)Show SMILES COc1ccc(Nc2c3cc(NC(=O)CCN4CCCC4)ccc3nc3ccc(NC(=O)CCN4CCCC4)cc23)cc1 Show InChI InChI=1S/C34H40N6O3/c1-43-27-10-6-24(7-11-27)37-34-28-22-25(35-32(41)14-20-39-16-2-3-17-39)8-12-30(28)38-31-13-9-26(23-29(31)34)36-33(42)15-21-40-18-4-5-19-40/h6-13,22-23H,2-5,14-21H2,1H3,(H,35,41)(H,36,42)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134005

(CHEMBL137928 | N,N'-(9-(2-(dimethylamino)ethylamin...)Show SMILES CN(C)CCNc1c2cc(NC(=O)CCN3CCCC3)ccc2nc2ccc(NC(=O)CCN3CCCC3)cc12 Show InChI InChI=1S/C31H43N7O2/c1-36(2)20-13-32-31-25-21-23(33-29(39)11-18-37-14-3-4-15-37)7-9-27(25)35-28-10-8-24(22-26(28)31)34-30(40)12-19-38-16-5-6-17-38/h7-10,21-22H,3-6,11-20H2,1-2H3,(H,32,35)(H,33,39)(H,34,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134001
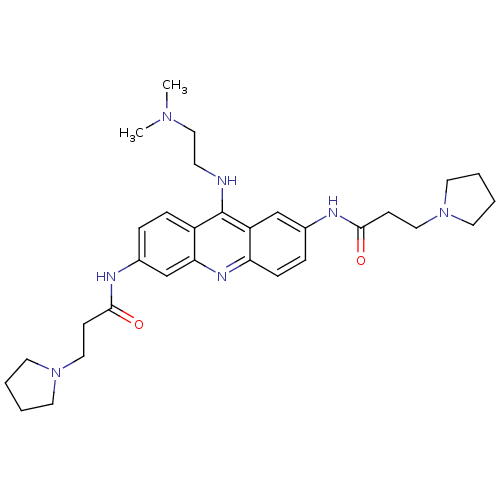
(CHEMBL140084 | N,N'-(9-(2-(dimethylamino)ethylamin...)Show SMILES CN(C)CCNc1c2ccc(NC(=O)CCN3CCCC3)cc2nc2ccc(NC(=O)CCN3CCCC3)cc12 Show InChI InChI=1S/C31H43N7O2/c1-36(2)20-13-32-31-25-9-7-24(34-30(40)12-19-38-16-5-6-17-38)22-28(25)35-27-10-8-23(21-26(27)31)33-29(39)11-18-37-14-3-4-15-37/h7-10,21-22H,3-6,11-20H2,1-2H3,(H,32,35)(H,33,39)(H,34,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134034
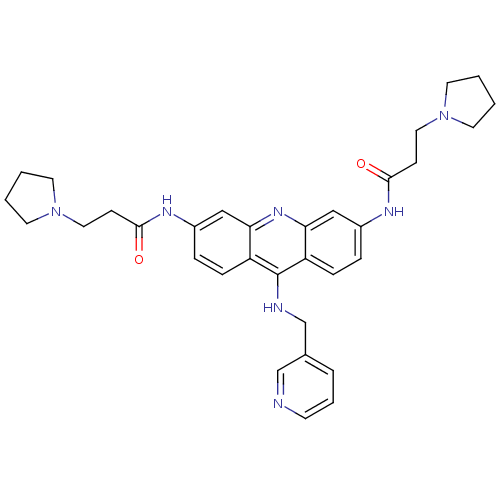
(CHEMBL139687 | N-[9-[(Pyridin-3-ylmethyl)-amino]-6...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2c(NCc3cccnc3)c3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C33H39N7O2/c41-31(11-18-39-14-1-2-15-39)36-25-7-9-27-29(20-25)38-30-21-26(37-32(42)12-19-40-16-3-4-17-40)8-10-28(30)33(27)35-23-24-6-5-13-34-22-24/h5-10,13,20-22H,1-4,11-12,14-19,23H2,(H,35,38)(H,36,41)(H,37,42) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibitory activity against human telomerase |
J Med Chem 46: 4463-76 (2003)
Article DOI: 10.1021/jm0308693
BindingDB Entry DOI: 10.7270/Q2RR1XN3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180764
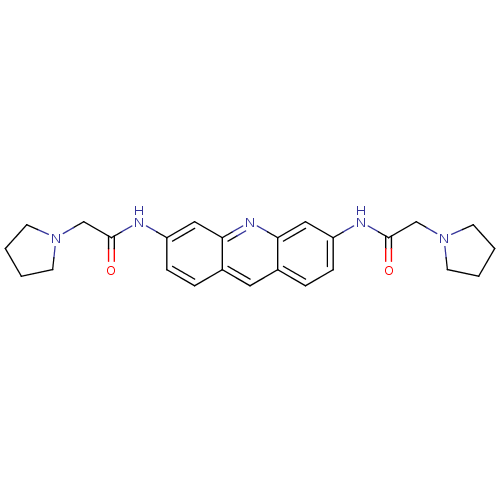
(2-pyrrolidin-1-yl-N-[6-(2-pyrrolidin-1-yl-acetylam...)Show SMILES O=C(CN1CCCC1)Nc1ccc2cc3ccc(NC(=O)CN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C25H29N5O2/c31-24(16-29-9-1-2-10-29)26-20-7-5-18-13-19-6-8-21(15-23(19)28-22(18)14-20)27-25(32)17-30-11-3-4-12-30/h5-8,13-15H,1-4,9-12,16-17H2,(H,26,31)(H,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180765
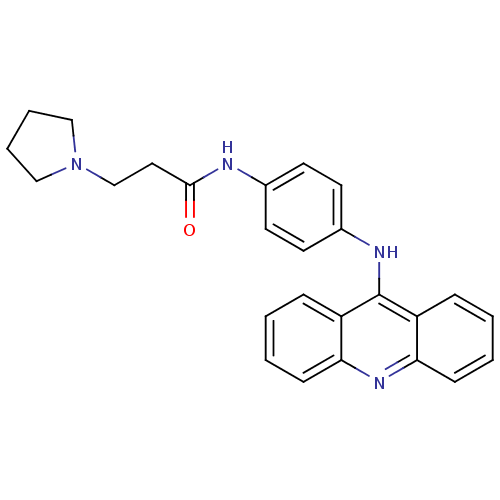
(CHEMBL381966 | N-[4-(acridin-9-ylamino)-phenyl]-3-...)Show SMILES O=C(CCN1CCCC1)Nc1ccc(Nc2c3ccccc3nc3ccccc23)cc1 Show InChI InChI=1S/C26H26N4O/c31-25(15-18-30-16-5-6-17-30)27-19-11-13-20(14-12-19)28-26-21-7-1-3-9-23(21)29-24-10-4-2-8-22(24)26/h1-4,7-14H,5-6,15-18H2,(H,27,31)(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180767
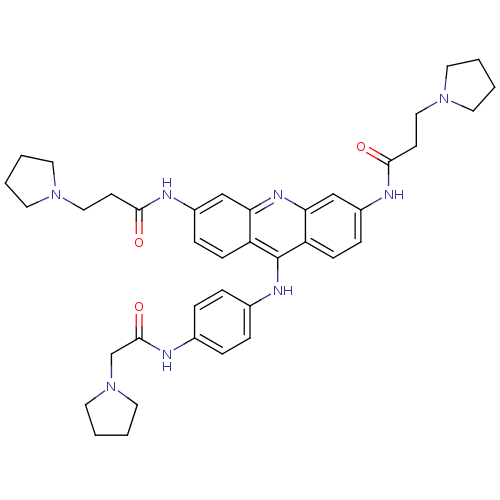
(3,6-bis[3-(pyrrolidin-1-yl)propionamido]-9-{4'-[2'...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CN4CCCC4)cc3)c3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C39H48N8O3/c48-36(15-23-45-17-1-2-18-45)41-30-11-13-32-34(25-30)44-35-26-31(42-37(49)16-24-46-19-3-4-20-46)12-14-33(35)39(32)43-29-9-7-28(8-10-29)40-38(50)27-47-21-5-6-22-47/h7-14,25-26H,1-6,15-24,27H2,(H,40,50)(H,41,48)(H,42,49)(H,43,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134030

(9-[4-(N,N-dimethylamino)phenylamino]-3,6-bis(3-pyr...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-13-9-26(36-33(43)15-21-41-17-3-4-18-41)23-31(29)39-32-24-27(10-14-30(32)35)37-34(44)16-22-42-19-5-6-20-42/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180770
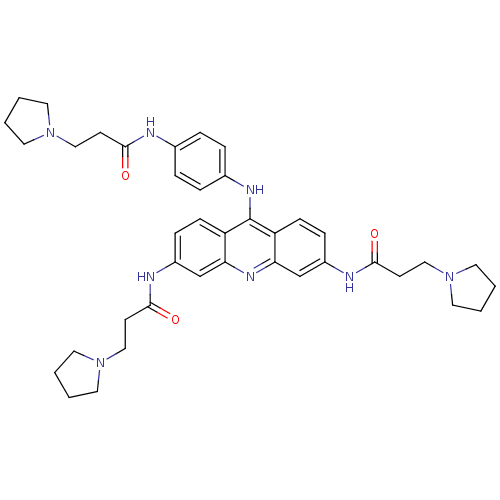
(3,6-bis[3-(pyrrolidin-1-yl)propionamido]-9-{4'-[3'...)Show SMILES O=C(CCN1CCCC1)Nc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C40H50N8O3/c49-37(15-24-46-18-1-2-19-46)41-29-7-9-30(10-8-29)44-40-33-13-11-31(42-38(50)16-25-47-20-3-4-21-47)27-35(33)45-36-28-32(12-14-34(36)40)43-39(51)17-26-48-22-5-6-23-48/h7-14,27-28H,1-6,15-26H2,(H,41,49)(H,42,50)(H,43,51)(H,44,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180763
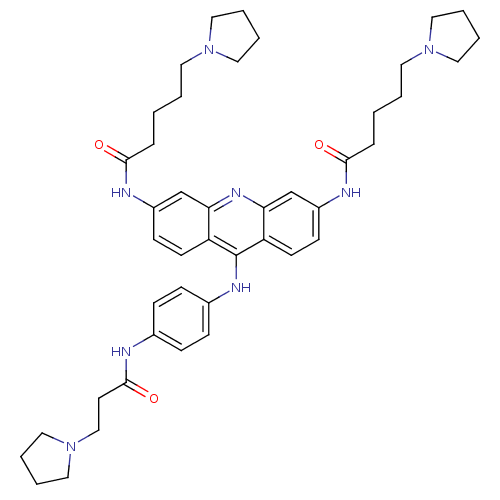
(3,6-bis[5-(pyrrolidin-1-yl)pentanamido]-9-{4'-[3''...)Show SMILES O=C(CCCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C44H58N8O3/c53-41(11-1-3-22-50-24-5-6-25-50)46-35-17-19-37-39(31-35)49-40-32-36(47-42(54)12-2-4-23-51-26-7-8-27-51)18-20-38(40)44(37)48-34-15-13-33(14-16-34)45-43(55)21-30-52-28-9-10-29-52/h13-20,31-32H,1-12,21-30H2,(H,45,55)(H,46,53)(H,47,54)(H,48,49) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 255 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180766

(3,6-bis[4-(pyrrolidin-1-yl)butanamido]-9-{4'-[3''-...)Show SMILES O=C(CCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C42H54N8O3/c51-39(9-7-26-48-20-1-2-21-48)44-33-15-17-35-37(29-33)47-38-30-34(45-40(52)10-8-27-49-22-3-4-23-49)16-18-36(38)42(35)46-32-13-11-31(12-14-32)43-41(53)19-28-50-24-5-6-25-50/h11-18,29-30H,1-10,19-28H2,(H,43,53)(H,44,51)(H,45,52)(H,46,47) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 326 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
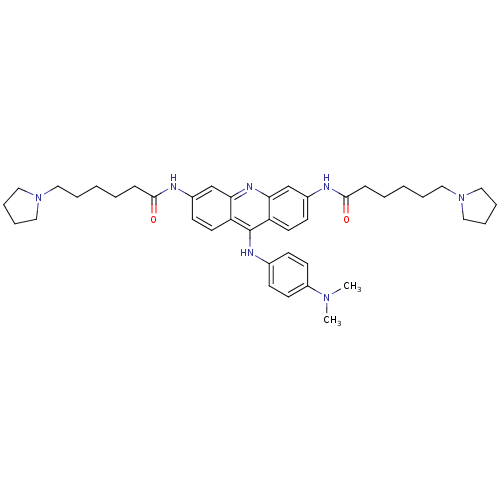
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180771
(3,6-bis[6-(pyrrolidin-1-yl)hexanamido]-9-[4'-(N,N-...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCCCCN4CCCC4)cc3nc3cc(NC(=O)CCCCCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C41H55N7O2/c1-46(2)34-19-15-31(16-20-34)44-41-35-21-17-32(42-39(49)13-5-3-7-23-47-25-9-10-26-47)29-37(35)45-38-30-33(18-22-36(38)41)43-40(50)14-6-4-8-24-48-27-11-12-28-48/h15-22,29-30H,3-14,23-28H2,1-2H3,(H,42,49)(H,43,50)(H,44,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.91E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
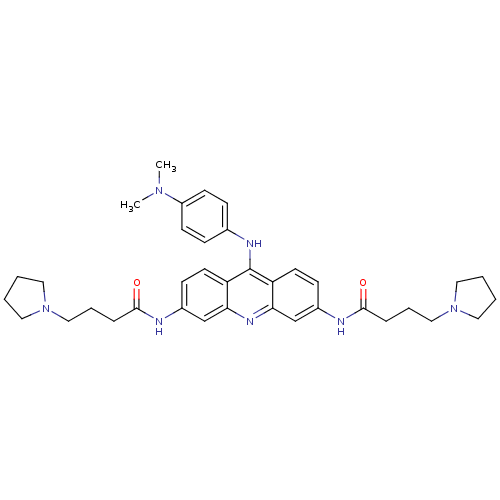
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180768
(3,6-[4-(pyrrolidin-1-yl)butanamido]-9-[4'-(N,N-dim...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCCN4CCCC4)cc3nc3cc(NC(=O)CCCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C37H47N7O2/c1-42(2)30-15-11-27(12-16-30)40-37-31-17-13-28(38-35(45)9-7-23-43-19-3-4-20-43)25-33(31)41-34-26-29(14-18-32(34)37)39-36(46)10-8-24-44-21-5-6-22-44/h11-18,25-26H,3-10,19-24H2,1-2H3,(H,38,45)(H,39,46)(H,40,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
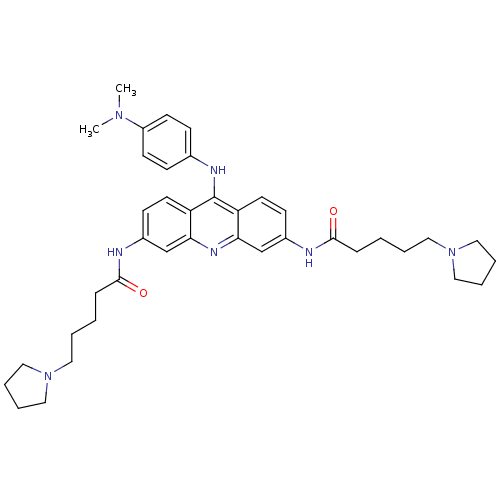
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180769
(3,6-bis[5-(pyrrolidin-1-yl)pentanamido]-9-[4'-(N,N...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCCCN4CCCC4)cc3nc3cc(NC(=O)CCCCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C39H51N7O2/c1-44(2)32-17-13-29(14-18-32)42-39-33-19-15-30(40-37(47)11-3-5-21-45-23-7-8-24-45)27-35(33)43-36-28-31(16-20-34(36)39)41-38(48)12-4-6-22-46-25-9-10-26-46/h13-20,27-28H,3-12,21-26H2,1-2H3,(H,40,47)(H,41,48)(H,42,43) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180772
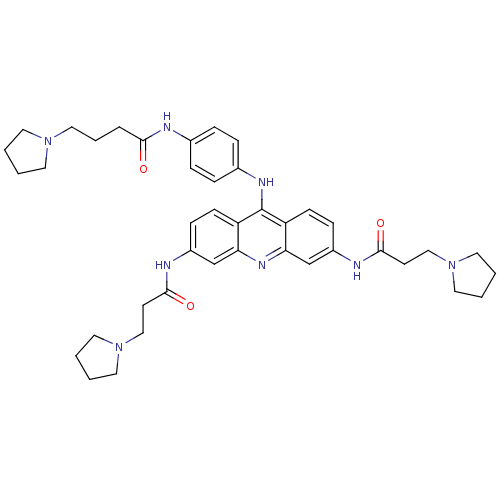
(3,6-bis[3-(pyrrolidin-1-yl)propionamido]-9-{4'-[3'...)Show SMILES O=C(CCCN1CCCC1)Nc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C41H52N8O3/c50-38(8-7-25-47-19-1-2-20-47)42-30-9-11-31(12-10-30)45-41-34-15-13-32(43-39(51)17-26-48-21-3-4-22-48)28-36(34)46-37-29-33(14-16-35(37)41)44-40(52)18-27-49-23-5-6-24-49/h9-16,28-29H,1-8,17-27H2,(H,42,50)(H,43,51)(H,44,52)(H,45,46) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180773
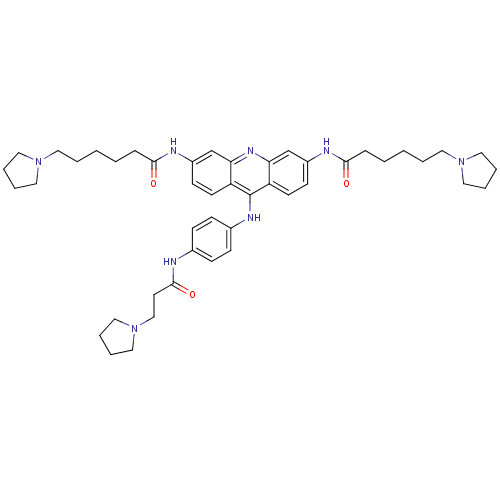
(3,6-bis[6-(pyrrolidin-1-yl)hexanamido]-9-{4'-[3''-...)Show SMILES O=C(CCCCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCCCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C46H62N8O3/c55-43(13-3-1-5-24-52-26-7-8-27-52)48-37-19-21-39-41(33-37)51-42-34-38(49-44(56)14-4-2-6-25-53-28-9-10-29-53)20-22-40(42)46(39)50-36-17-15-35(16-18-36)47-45(57)23-32-54-30-11-12-31-54/h15-22,33-34H,1-14,23-32H2,(H,47,57)(H,48,55)(H,49,56)(H,50,51) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 146 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134030

(9-[4-(N,N-dimethylamino)phenylamino]-3,6-bis(3-pyr...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-13-9-26(36-33(43)15-21-41-17-3-4-18-41)23-31(29)39-32-24-27(10-14-30(32)35)37-34(44)16-22-42-19-5-6-20-42/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase (unknown origin) by TRAP-LIG assay |
J Med Chem 51: 7751-67 (2008)
Article DOI: 10.1021/jm801245v
BindingDB Entry DOI: 10.7270/Q2CN73SS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data