

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642537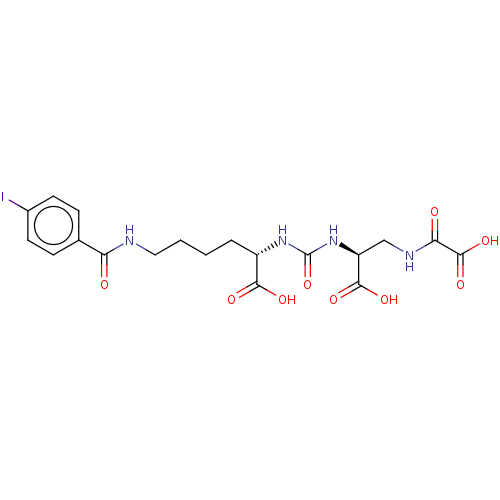 (US20230414794, Compound S2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481609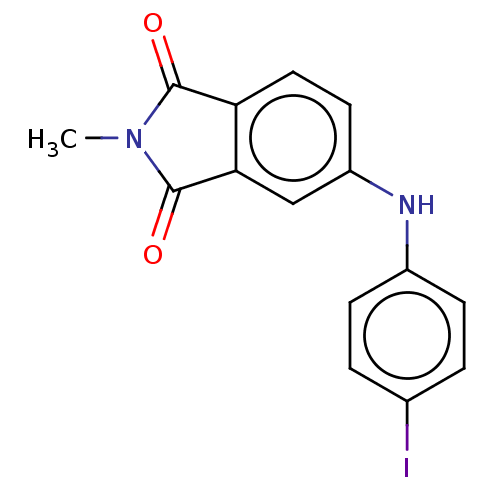 (CHEMBL610504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50304738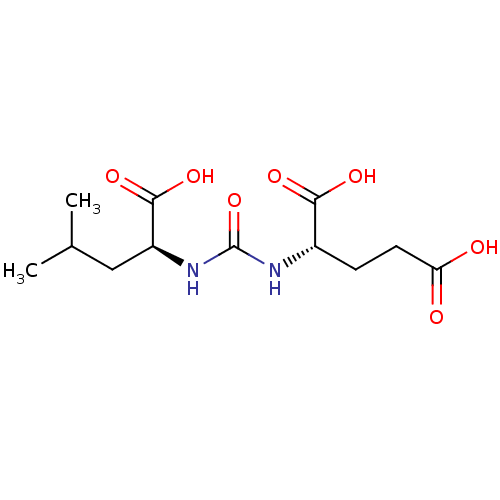 (2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481608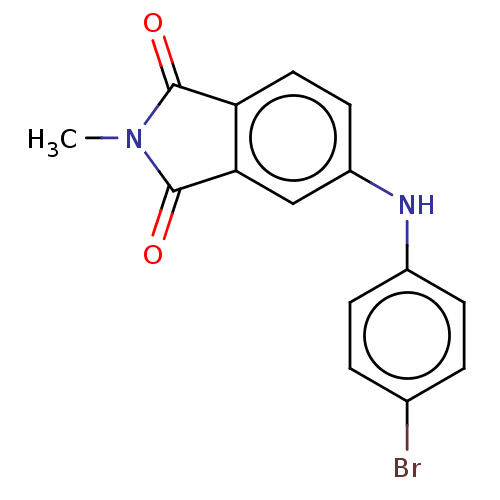 (CHEMBL598081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481605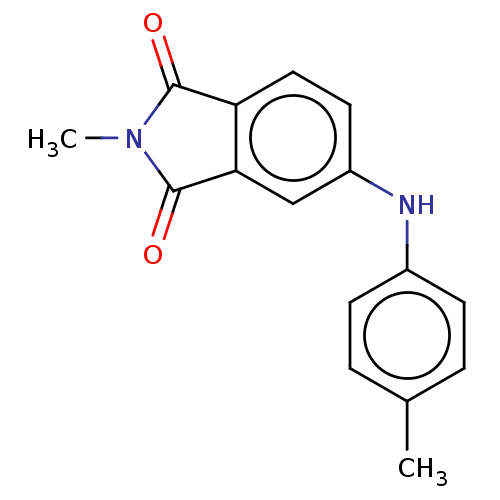 (CHEMBL598288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642538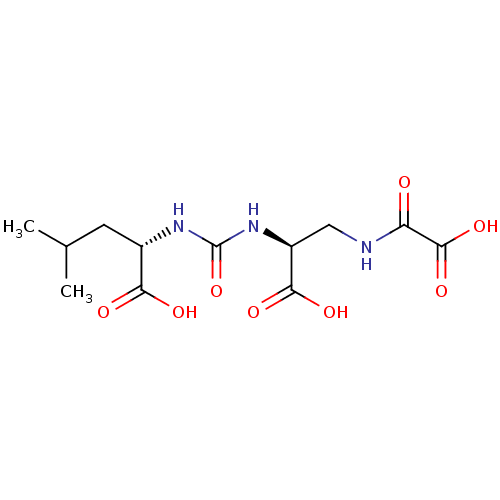 (US20230414794, Compound S3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 5.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642536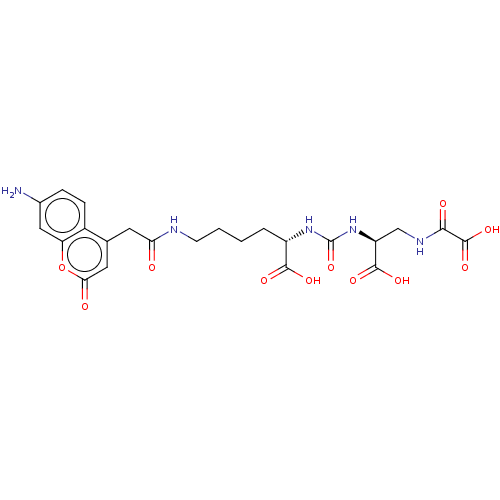 (US20230414794, Compound S1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 5.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481604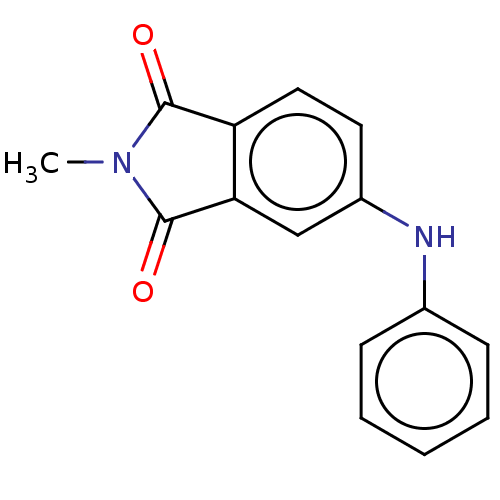 (CHEMBL610505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481606 (CHEMBL598082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481607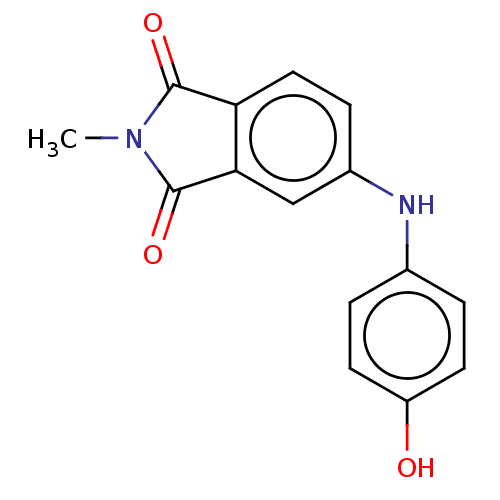 (CHEMBL598083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642541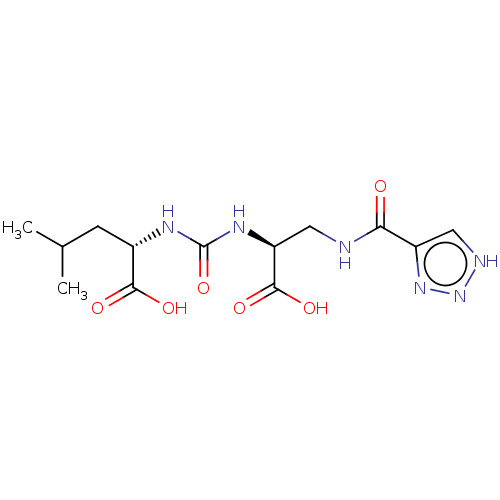 (US20230414794, Comparative Compound DS3 | US202304...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642540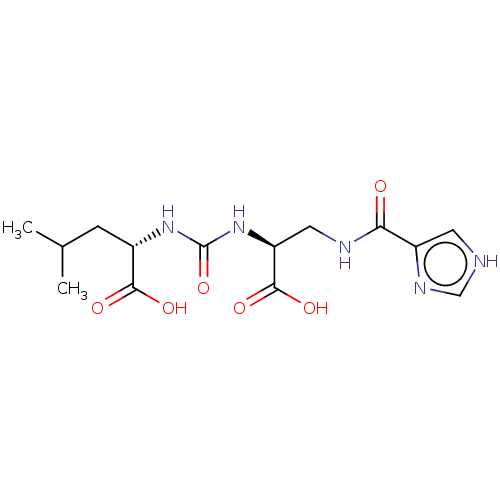 (US20230414794, Comparative Compound DS2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642539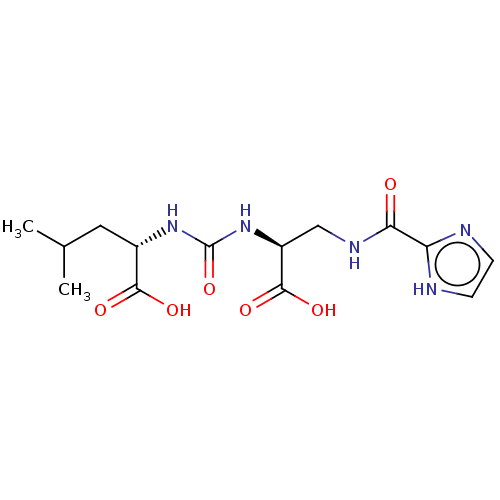 (US20230414794, Comparative Compound DS1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50100134 (2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50040929 (4,5-dianilinophthalimide | 5,6-bis(phenylamino)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50122787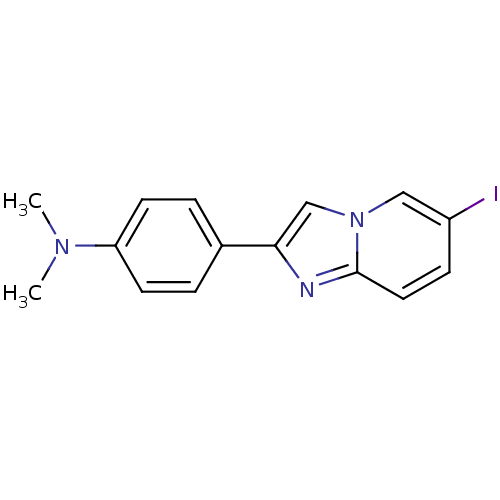 (2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793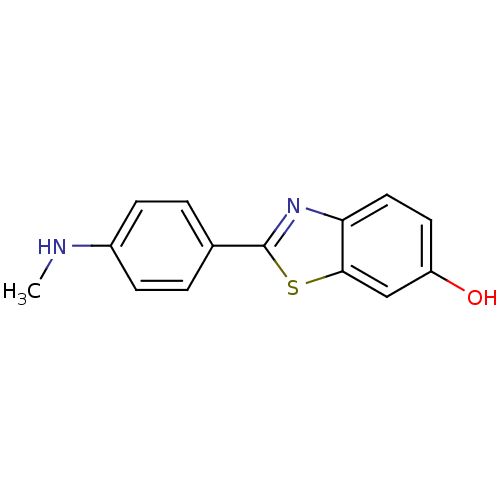 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642541 (US20230414794, Comparative Compound DS3 | US202304...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50040923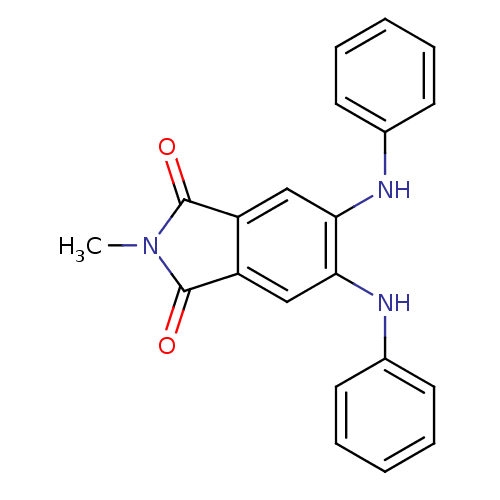 (2-Methyl-5,6-bis-phenylamino-isoindole-1,3-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50079267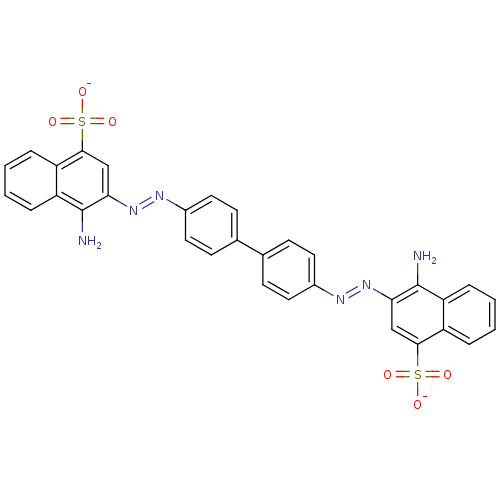 (Congo Red | Direct red 28 | Kongorot | Sodium diph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University Curated by ChEMBL | Assay Description Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain | Bioorg Med Chem 18: 1337-43 (2010) Article DOI: 10.1016/j.bmc.2009.12.023 BindingDB Entry DOI: 10.7270/Q2445Q9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515356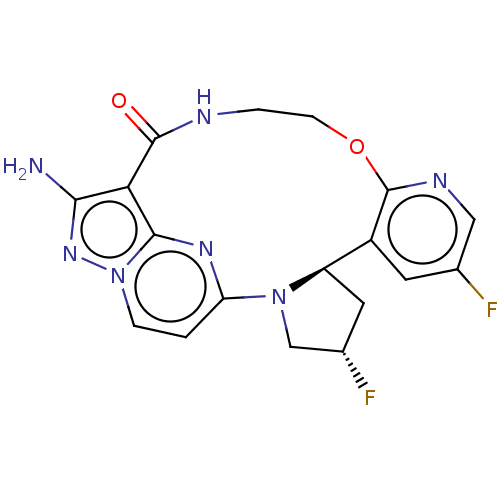 ((13E,14E,22R,24S)-12-amino-24,35- difluoro-4-oxa-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515368 ((13E,14E,22R,24S)-12-amino- 24,35-difluoro-7-aza-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515377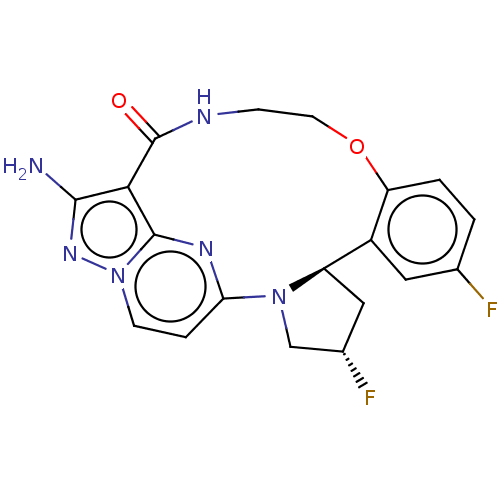 ((13E,14E,22R,24S)-12-amino- 24,35-difluoro-4-oxa-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515357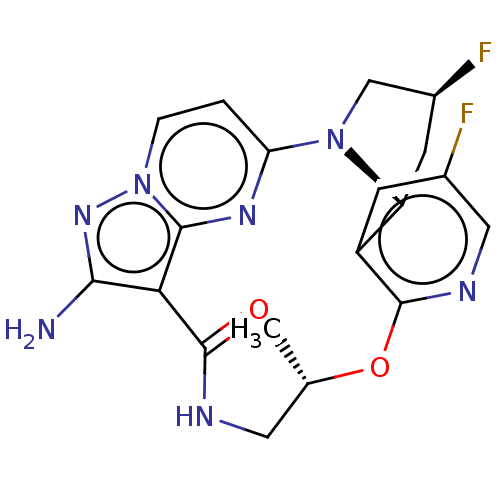 ((13E,14E,22R,24S,5S)-12-amino-24,35-difluoro- 5-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515365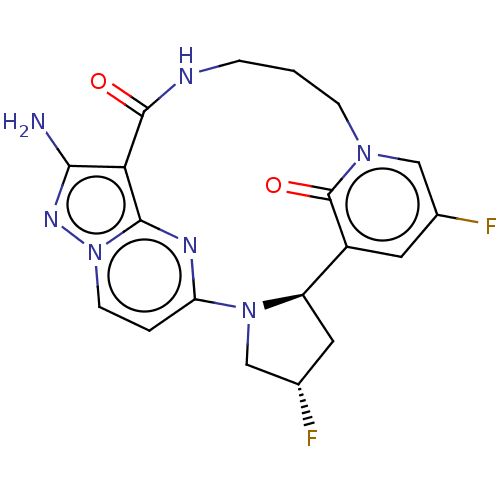 (US11098060, Example 23) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515367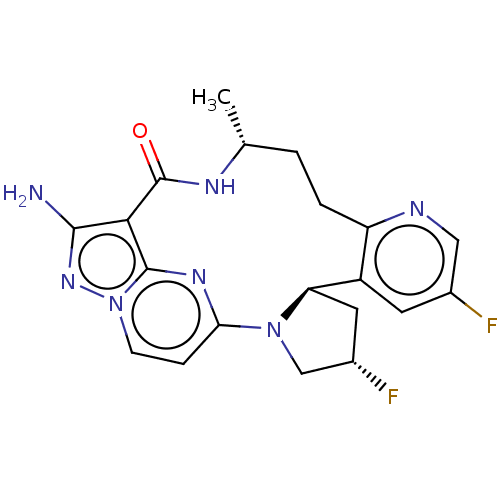 (US11098060, Example 26) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515370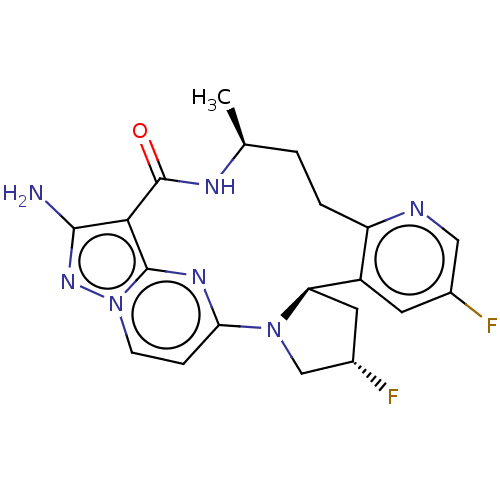 ((13E,14E,22R,24S,6S)-12-amino- 24,35-difluoro-6-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515369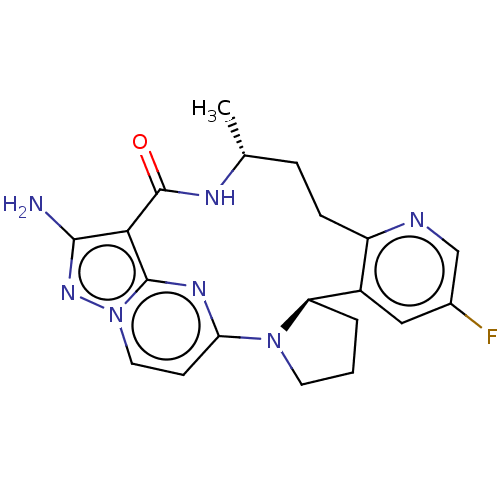 ((13E,14E,22R,6R)-12-amino-35- fluoro-6-methyl-7-az...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G595R] (Homo sapiens (Human)) | BDBM515354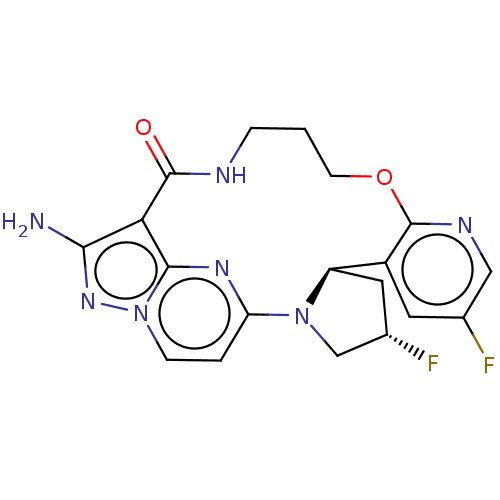 (US11098060, Example 12 | US11098060, Example 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG595R (Kinase domain) kinase was expressed in Sf9 cells using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515375 ((13E,14E,22R,24S)-12-amino-24,35,6,6- tetrafluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515348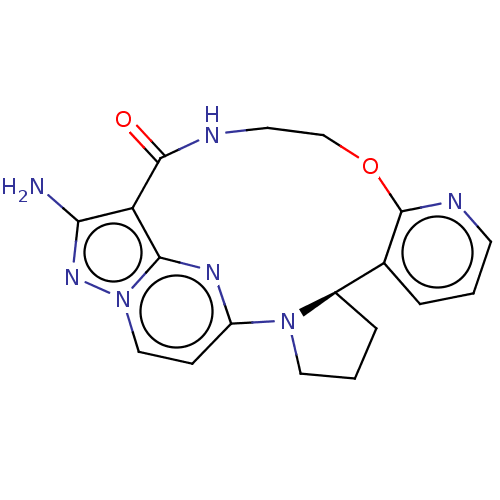 ((R,13E,14E)-12-amino-4-oxa-7-aza-1(5,3)- pyrazolo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515361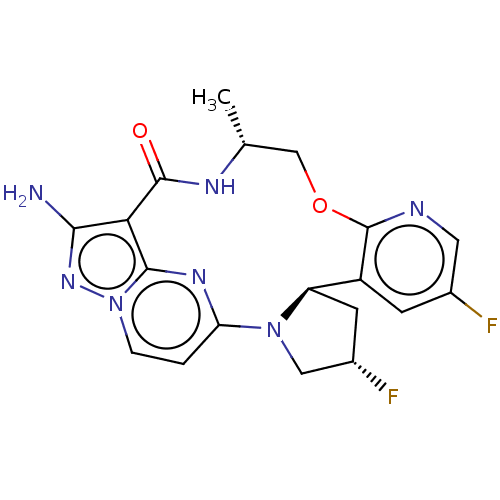 ((13E,14E,22R,24S,6R)-12-amino-24, 35-difluoro-6-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515363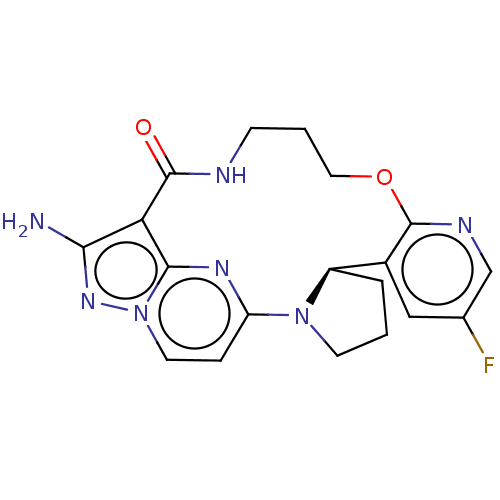 (US11098060, Example 21) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515360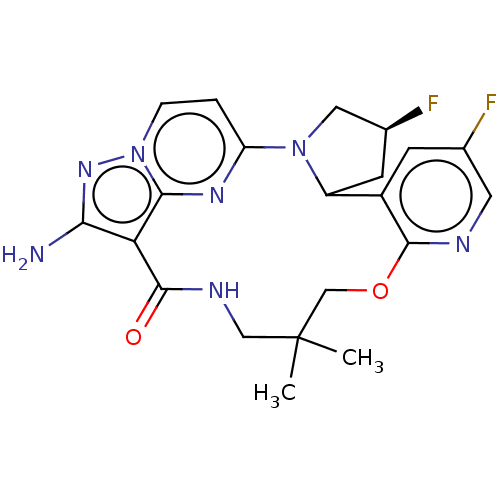 ((13E,14E,22R,24S)-12-amino-24,35-difluoro- 6,6-dim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G595R] (Homo sapiens (Human)) | BDBM515356 ((13E,14E,22R,24S)-12-amino-24,35- difluoro-4-oxa-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG595R (Kinase domain) kinase was expressed in Sf9 cells using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515350 ((R,13E,14E)-12-amino-35-fluro-4-oxa-7-aza- 1(5,3)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515372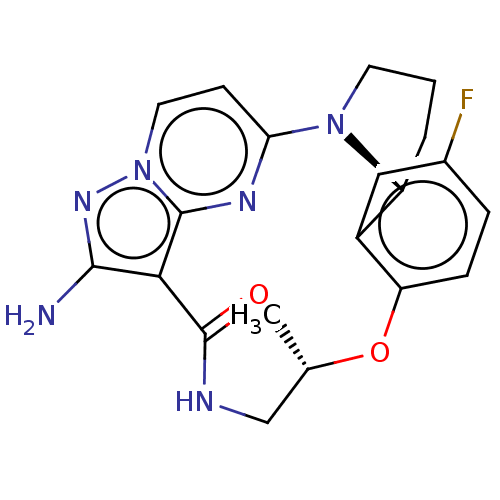 ((13E,14E,22R,5S)-12-amino-35- fluoro-5-methyl-4-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515351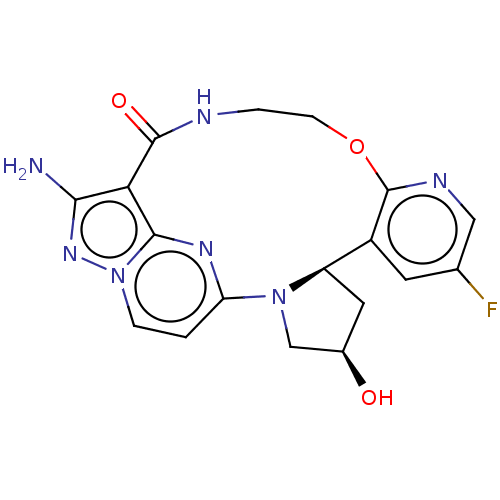 ((13E,14E,22R,24R)-12-amino-35fluoro-24- hydroxy-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515357 ((13E,14E,22R,24S,5S)-12-amino-24,35-difluoro- 5-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515349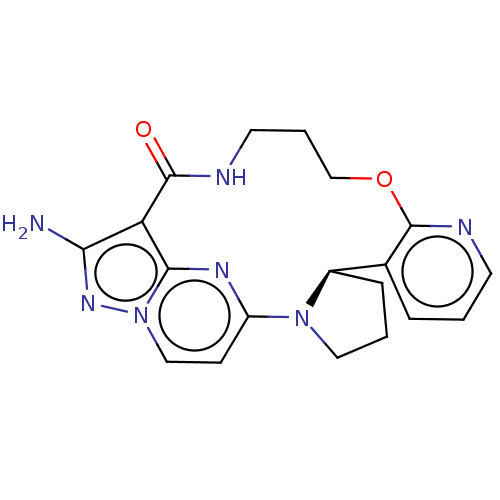 ((R,13E,14E)-12-amino-4-oxa-8-aza-1(5,3)- pyrazolo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50608288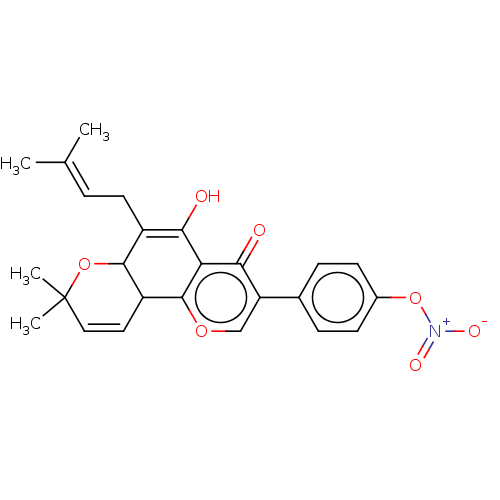 (CHEMBL5278739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515358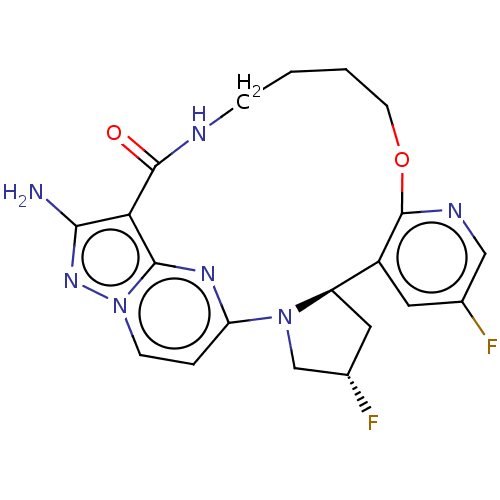 ((13E,14E,22R,24S)-12-amino-24,35-difluoro- 4-oxa-9...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G595R] (Homo sapiens (Human)) | BDBM515357 ((13E,14E,22R,24S,5S)-12-amino-24,35-difluoro- 5-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG595R (Kinase domain) kinase was expressed in Sf9 cells using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G667C] (Homo sapiens (Human)) | BDBM515358 ((13E,14E,22R,24S)-12-amino-24,35-difluoro- 4-oxa-9...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515364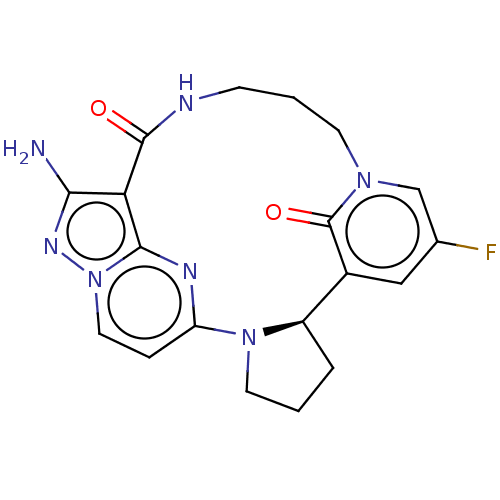 (US11098060, Example 22) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515346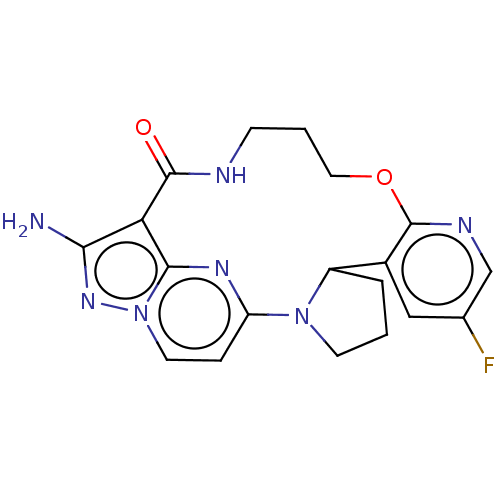 ((13E,14E)-12-amino-35-fluoro-4-oxa-8-aza-1 (5,3)-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515359 ((13E,14E,22R,24S)-12-amino-24,35-difluoro- 8-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515355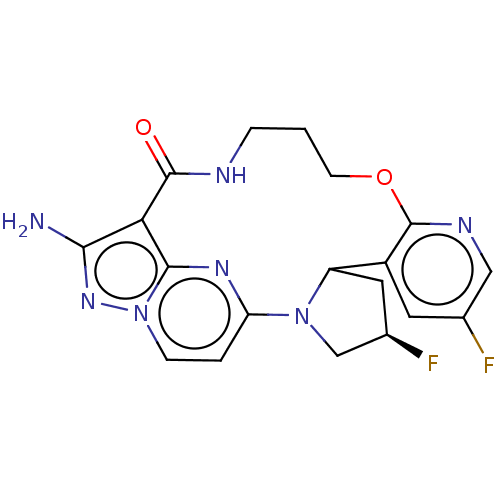 ((13E,14E, 22R,24R)-12-amino-24,35- difluoro-4-oxa-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM515356 ((13E,14E,22R,24S)-12-amino-24,35- difluoro-4-oxa-7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor [G595R] (Homo sapiens (Human)) | BDBM515367 (US11098060, Example 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TrkAG595R (Kinase domain) kinase was expressed in Sf9 cells using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 368 total ) | Next | Last >> |