 Found 526 hits with Last Name = 'zhang' and Initial = 'xj'
Found 526 hits with Last Name = 'zhang' and Initial = 'xj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora A (62 to 344 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Emopamil-binding protein-like
(Homo sapiens (Human)) | BDBM50424041
(CHEMBL2314420)Show InChI InChI=1S/C22H30N2O3/c1-26-21-10-9-20(18-22(21)27-25)11-13-24-16-14-23(15-17-24)12-5-8-19-6-3-2-4-7-19/h2-4,6-7,9-10,18,25H,5,8,11-17H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Binding affinity to emopamil binding protein (unknown origin) |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50424039

(CHEMBL2314424)Show InChI InChI=1S/C21H25FN2O3/c22-7-12-25-19-4-1-17(2-5-19)14-23-8-10-24(11-9-23)15-18-3-6-20-21(13-18)27-16-26-20/h1-6,13H,7-12,14-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma1 receptor in rat brain membranes |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041
(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Emopamil-binding protein-like
(Homo sapiens (Human)) | BDBM20607
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Binding affinity to emopamil binding protein (unknown origin) |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of G9a (unknown origin) by Morrison plot analysis in presence of histone H3 (1 to 25 residues) |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Emopamil-binding protein-like
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Binding affinity to emopamil binding protein (unknown origin) |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma1 receptor in rat brain membranes |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged Aurora B (1 to 403 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora B (1 to 403 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50424038

(CHEMBL2314425)Show InChI InChI=1S/C23H29FN2O4/c24-7-12-27-13-14-28-21-4-1-19(2-5-21)16-25-8-10-26(11-9-25)17-20-3-6-22-23(15-20)30-18-29-22/h1-6,15H,7-14,16-18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma1 receptor in rat brain membranes |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Vesicular acetylcholine transporter
(Homo sapiens (Human)) | BDBM50334158

(1'-benzyl-3-(2-fluoroethyl)-3H-spiro[[2]benzofuran...)Show InChI InChI=1S/C21H24FNO/c22-13-10-20-18-8-4-5-9-19(18)21(24-20)11-14-23(15-12-21)16-17-6-2-1-3-7-17/h1-9,20H,10-16H2 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Binding affinity to VAChT (unknown origin) |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50424037

(CHEMBL2314426)Show SMILES FCCOCCOCCOc1ccc(CN2CCN(Cc3ccc4OCOc4c3)CC2)cc1 Show InChI InChI=1S/C25H33FN2O5/c26-7-12-29-13-14-30-15-16-31-23-4-1-21(2-5-23)18-27-8-10-28(11-9-27)19-22-3-6-24-25(17-22)33-20-32-24/h1-6,17H,7-16,18-20H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma1 receptor in rat brain membranes |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Emopamil-binding protein-like
(Homo sapiens (Human)) | BDBM50334158

(1'-benzyl-3-(2-fluoroethyl)-3H-spiro[[2]benzofuran...)Show InChI InChI=1S/C21H24FNO/c22-13-10-20-18-8-4-5-9-19(18)21(24-20)11-14-23(15-12-21)16-17-6-2-1-3-7-17/h1-9,20H,10-16H2 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Binding affinity to emopamil binding protein (unknown origin) |
Bioorg Med Chem 21: 215-22 (2012)
Article DOI: 10.1016/j.bmc.2012.10.038
BindingDB Entry DOI: 10.7270/Q2VM4DKP |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503744
(CHEMBL4563026)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(F)c(-[#7]-c3ccc(cc3)-[#6](=O)-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C33H34ClFN7O3/c1-32(2)17-24(18-33(3,4)42(32)45)38-29(43)20-11-15-23(16-12-20)39-31-36-19-26(35)28(41-31)37-22-13-9-21(10-14-22)30(44)40-27-8-6-5-7-25(27)34/h5-16,19,24H,17-18H2,1-4H3,(H,38,43)(H,40,44)(H2,36,37,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503745
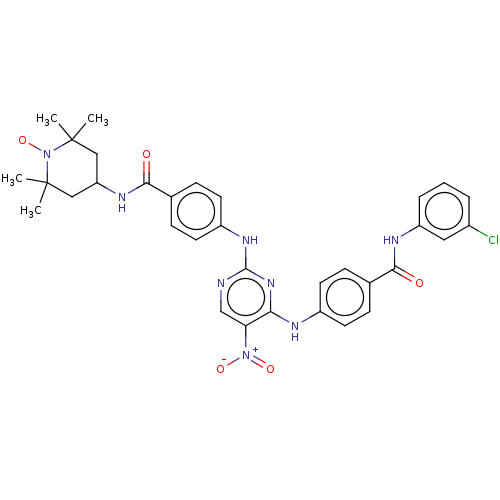
(CHEMBL4460717)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(cc3)-[#6](=O)-[#7]-c3cccc(Cl)c3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C33H34ClN8O5/c1-32(2)17-26(18-33(3,4)42(32)47)38-30(44)21-10-14-24(15-11-21)39-31-35-19-27(41(45)46)28(40-31)36-23-12-8-20(9-13-23)29(43)37-25-7-5-6-22(34)16-25/h5-16,19,26H,17-18H2,1-4H3,(H,37,43)(H,38,44)(H2,35,36,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503748
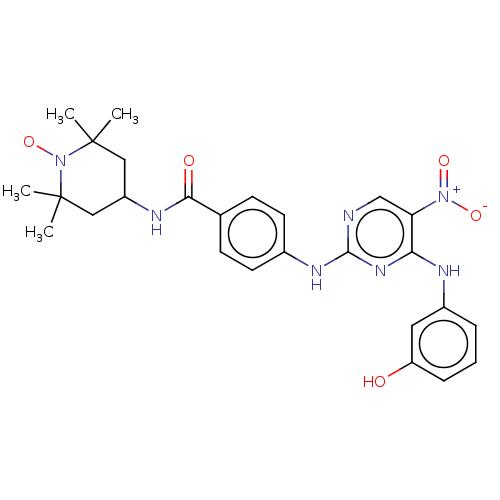
(CHEMBL4440617)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3cccc(-[#8])c3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H30N7O5/c1-25(2)13-19(14-26(3,4)33(25)38)29-23(35)16-8-10-17(11-9-16)30-24-27-15-21(32(36)37)22(31-24)28-18-6-5-7-20(34)12-18/h5-12,15,19,34H,13-14H2,1-4H3,(H,29,35)(H2,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503740
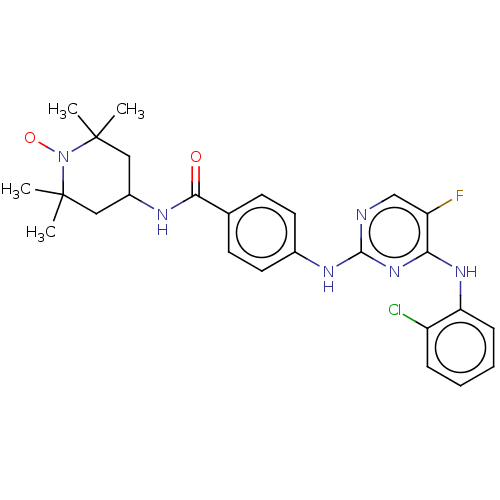
(CHEMBL4451940)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(F)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29ClFN6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503749
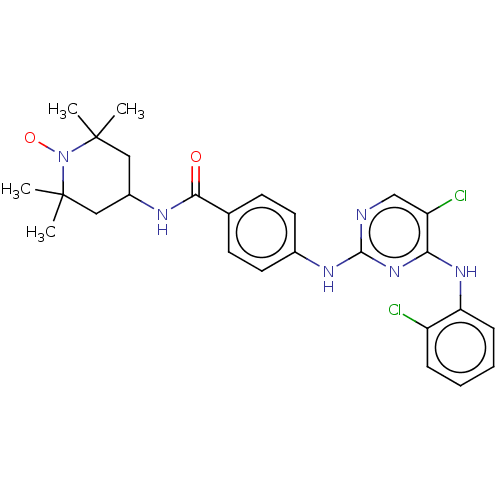
(CHEMBL4455202)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Cl)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29Cl2N6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503749

(CHEMBL4455202)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Cl)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29Cl2N6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503741
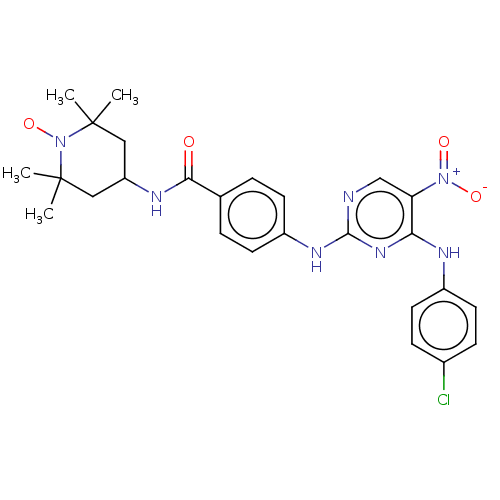
(CHEMBL4443125)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(Cl)cc3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H29ClN7O4/c1-25(2)13-20(14-26(3,4)34(25)38)30-23(35)16-5-9-19(10-6-16)31-24-28-15-21(33(36)37)22(32-24)29-18-11-7-17(27)8-12-18/h5-12,15,20H,13-14H2,1-4H3,(H,30,35)(H2,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50466808

(AZD-1152-HQPA | Barasertib)Show SMILES CCN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)[nH]n3)ncnc2c1 Show InChI InChI=1S/C26H30FN7O3/c1-2-34(10-11-35)9-4-12-37-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(36)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17,35H,2,4,9-12,15H2,1H3,(H,30,36)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503737
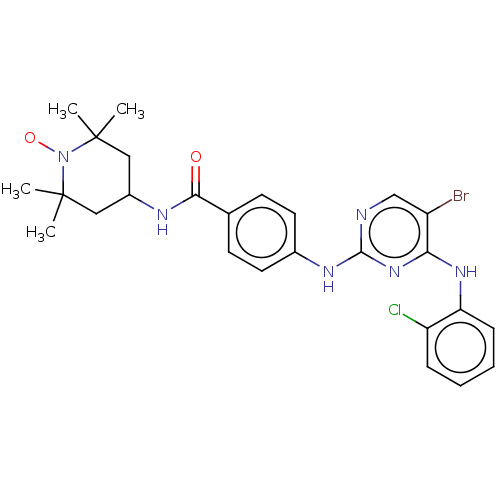
(CHEMBL4471448)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Br)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29BrClN6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-19(27)22(33-24)32-21-8-6-5-7-20(21)28/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503743
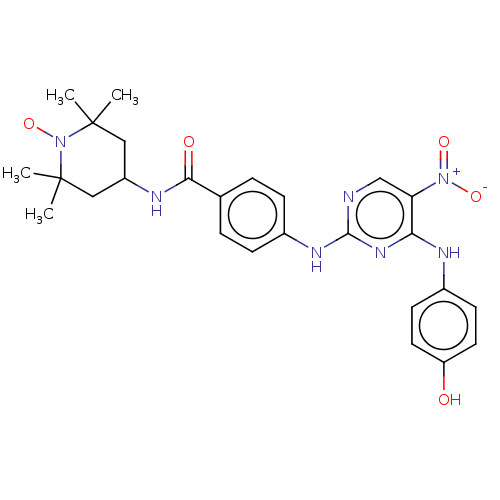
(CHEMBL4447241)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(-[#8])cc3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H30N7O5/c1-25(2)13-19(14-26(3,4)33(25)38)29-23(35)16-5-7-18(8-6-16)30-24-27-15-21(32(36)37)22(31-24)28-17-9-11-20(34)12-10-17/h5-12,15,19,34H,13-14H2,1-4H3,(H,29,35)(H2,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503739
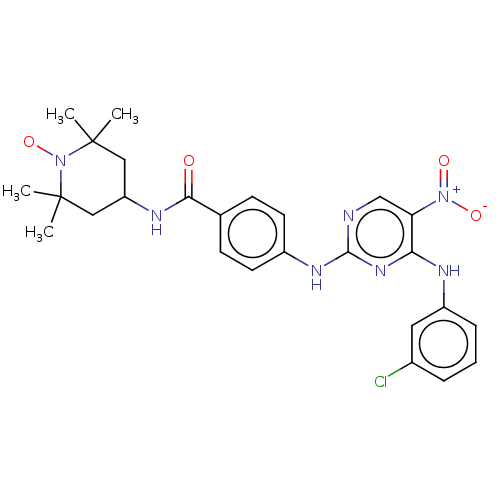
(CHEMBL4457980)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3cccc(Cl)c3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H29ClN7O4/c1-25(2)13-20(14-26(3,4)34(25)38)30-23(35)16-8-10-18(11-9-16)31-24-28-15-21(33(36)37)22(32-24)29-19-7-5-6-17(27)12-19/h5-12,15,20H,13-14H2,1-4H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503750
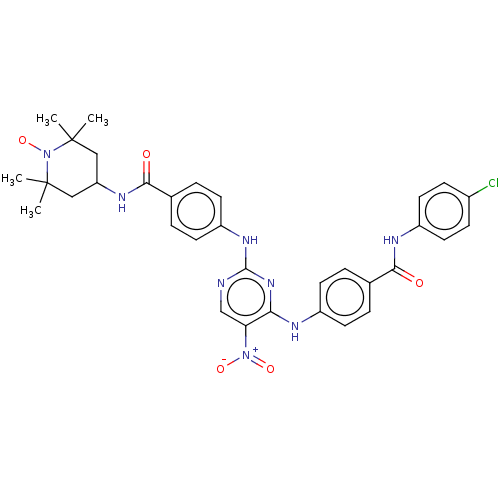
(CHEMBL4516770)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(cc3)-[#6](=O)-[#7]-c3ccc(Cl)cc3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C33H34ClN8O5/c1-32(2)17-26(18-33(3,4)42(32)47)38-30(44)21-7-13-25(14-8-21)39-31-35-19-27(41(45)46)28(40-31)36-23-11-5-20(6-12-23)29(43)37-24-15-9-22(34)10-16-24/h5-16,19,26H,17-18H2,1-4H3,(H,37,43)(H,38,44)(H2,35,36,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50466804
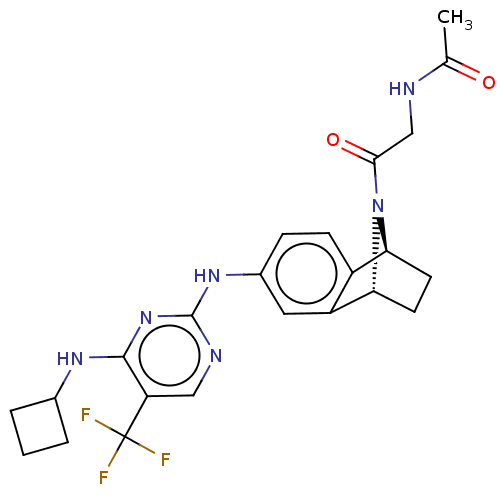
(CHEMBL4289017)Show SMILES [H][C@@]12CC[C@@]([H])(N1C(=O)CNC(C)=O)c1cc(Nc3ncc(c(NC4CCC4)n3)C(F)(F)F)ccc21 |r,TLB:7:6:14.35:2.3| Show InChI InChI=1S/C23H25F3N6O2/c1-12(33)27-11-20(34)32-18-7-8-19(32)16-9-14(5-6-15(16)18)30-22-28-10-17(23(24,25)26)21(31-22)29-13-3-2-4-13/h5-6,9-10,13,18-19H,2-4,7-8,11H2,1H3,(H,27,33)(H2,28,29,30,31)/t18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length His-tagged Aurora A (unknown origin) expressed in insect cells using biotinylated LRRWSLG as substrate in prese... |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50164787
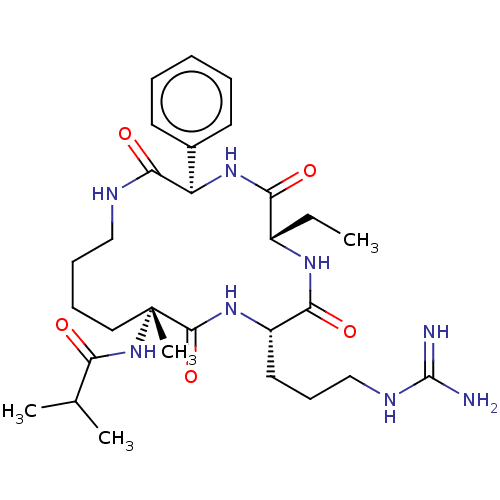
(CHEMBL3798088)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@](C)(CCCCNC(=O)[C@H](NC1=O)c1ccccc1)NC(=O)C(C)C |r| Show InChI InChI=1S/C29H46N8O5/c1-5-20-24(39)36-22(19-12-7-6-8-13-19)26(41)32-16-10-9-15-29(4,37-23(38)18(2)3)27(42)35-21(25(40)34-20)14-11-17-33-28(30)31/h6-8,12-13,18,20-22H,5,9-11,14-17H2,1-4H3,(H,32,41)(H,34,40)(H,35,42)(H,36,39)(H,37,38)(H4,30,31,33)/t20-,21-,22+,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of fluorescence-labeled Ac-ARA peptide binding to WDR5 (unknown origin) by fluorescence polarization assay |
Eur J Med Chem 124: 480-489 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.036
BindingDB Entry DOI: 10.7270/Q2251M6G |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) after 1 hr in presence of ATP by Kinase-Glo reagent-based luminescence assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503740

(CHEMBL4451940)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(F)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29ClFN6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503741

(CHEMBL4443125)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(Cl)cc3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H29ClN7O4/c1-25(2)13-20(14-26(3,4)34(25)38)30-23(35)16-5-9-19(10-6-16)31-24-28-15-21(33(36)37)22(32-24)29-18-11-7-17(27)8-12-18/h5-12,15,20H,13-14H2,1-4H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM200723

(US9233086, 10L)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C35H49F2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of 10mer-Thr-FAM probe binding to human WDR5 after 2 hrs by fluorescence polarization assay |
Eur J Med Chem 124: 480-489 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.036
BindingDB Entry DOI: 10.7270/Q2251M6G |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50277584
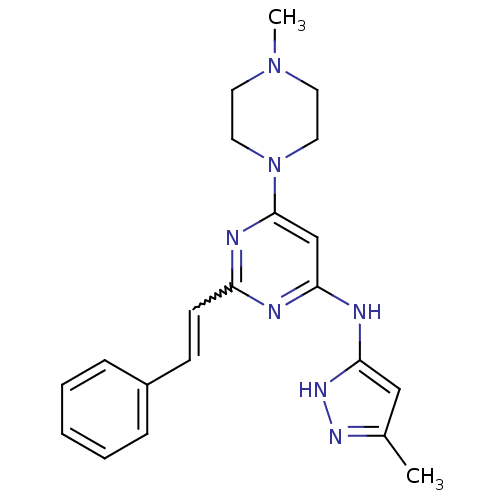
(CHEMBL482968 | N-(5-methyl-1H-pyrazol-3-yl)-6-(4-m...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(C=Cc2ccccc2)n1 |w:19.20| Show InChI InChI=1S/C21H25N7/c1-16-14-20(26-25-16)23-19-15-21(28-12-10-27(2)11-13-28)24-18(22-19)9-8-17-6-4-3-5-7-17/h3-9,14-15H,10-13H2,1-2H3,(H2,22,23,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLT3 |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503747
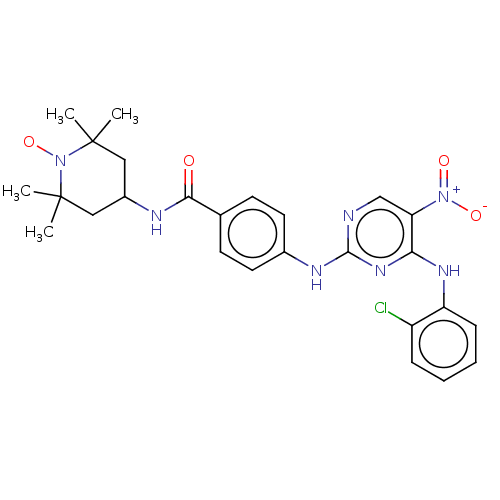
(CHEMBL4520619)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccccc3Cl)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H29ClN7O4/c1-25(2)13-18(14-26(3,4)34(25)38)29-23(35)16-9-11-17(12-10-16)30-24-28-15-21(33(36)37)22(32-24)31-20-8-6-5-7-19(20)27/h5-12,15,18H,13-14H2,1-4H3,(H,29,35)(H2,28,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503746
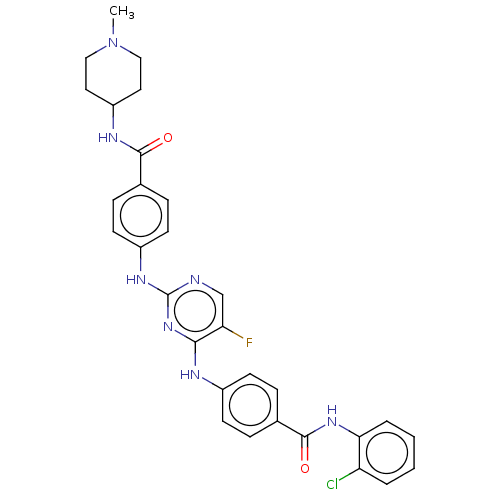
(CHEMBL4528809)Show SMILES CN1CCC(CC1)NC(=O)c1ccc(Nc2ncc(F)c(Nc3ccc(cc3)C(=O)Nc3ccccc3Cl)n2)cc1 Show InChI InChI=1S/C30H29ClFN7O2/c1-39-16-14-23(15-17-39)35-28(40)19-8-12-22(13-9-19)36-30-33-18-25(32)27(38-30)34-21-10-6-20(7-11-21)29(41)37-26-5-3-2-4-24(26)31/h2-13,18,23H,14-17H2,1H3,(H,35,40)(H,37,41)(H2,33,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM200723

(US9233086, 10L)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C35H49F2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of fluorescence-labeled Ac-ARA peptide binding to WDR5 (unknown origin) by fluorescence polarization assay |
Eur J Med Chem 124: 480-489 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.036
BindingDB Entry DOI: 10.7270/Q2251M6G |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25933
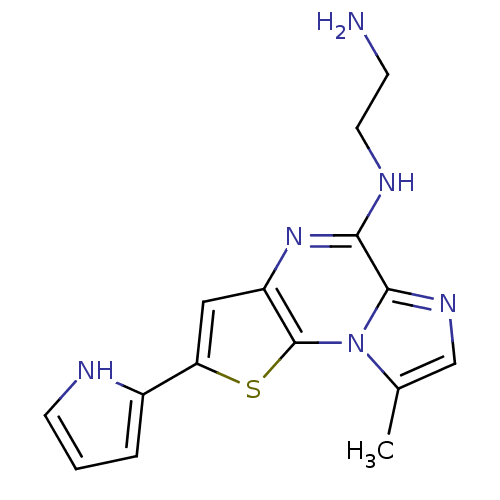
(N-(2-aminoethyl)-12-methyl-4-(1H-pyrrol-2-yl)-3-th...)Show InChI InChI=1S/C15H16N6S/c1-9-8-19-14-13(18-6-4-16)20-11-7-12(10-3-2-5-17-10)22-15(11)21(9)14/h2-3,5,7-8,17H,4,6,16H2,1H3,(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human IKKbeta using GST-IkappaBalpha as substrate |
Eur J Med Chem 63: 269-78 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.045
BindingDB Entry DOI: 10.7270/Q2XG9SHG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50442103
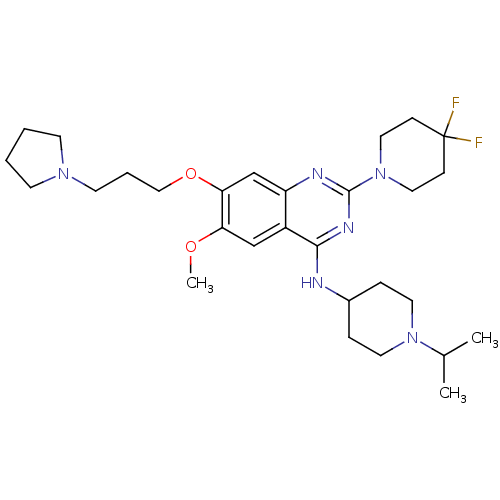
(CHEMBL2441082)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)N1CCC(F)(F)CC1 Show InChI InChI=1S/C29H44F2N6O2/c1-21(2)36-14-7-22(8-15-36)32-27-23-19-25(38-3)26(39-18-6-13-35-11-4-5-12-35)20-24(23)33-28(34-27)37-16-9-29(30,31)10-17-37/h19-22H,4-18H2,1-3H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503744

(CHEMBL4563026)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(F)c(-[#7]-c3ccc(cc3)-[#6](=O)-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C33H34ClFN7O3/c1-32(2)17-24(18-33(3,4)42(32)45)38-29(43)20-11-15-23(16-12-20)39-31-36-19-26(35)28(41-31)37-22-13-9-21(10-14-22)30(44)40-27-8-6-5-7-25(27)34/h5-16,19,24H,17-18H2,1-4H3,(H,38,43)(H,40,44)(H2,36,37,39,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503737

(CHEMBL4471448)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Br)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29BrClN6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-19(27)22(33-24)32-21-8-6-5-7-20(21)28/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25960
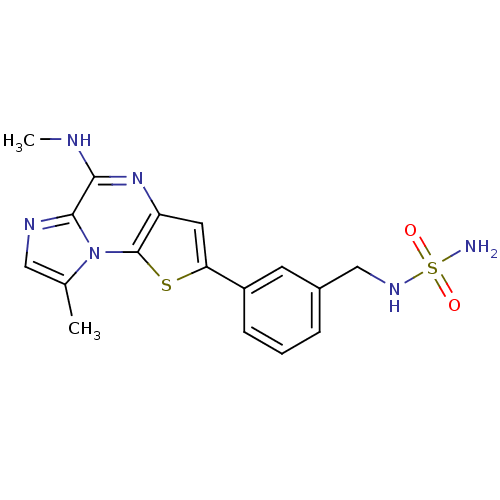
(amino-N-({3-[12-methyl-8-(methylamino)-3-thia-1,7,...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CNS(N)(=O)=O)c1 Show InChI InChI=1S/C17H18N6O2S2/c1-10-8-20-16-15(19-2)22-13-7-14(26-17(13)23(10)16)12-5-3-4-11(6-12)9-21-27(18,24)25/h3-8,21H,9H2,1-2H3,(H,19,22)(H2,18,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human IKKbeta using GST-IkappaBalpha as substrate |
Eur J Med Chem 63: 269-78 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.045
BindingDB Entry DOI: 10.7270/Q2XG9SHG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data