

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50001922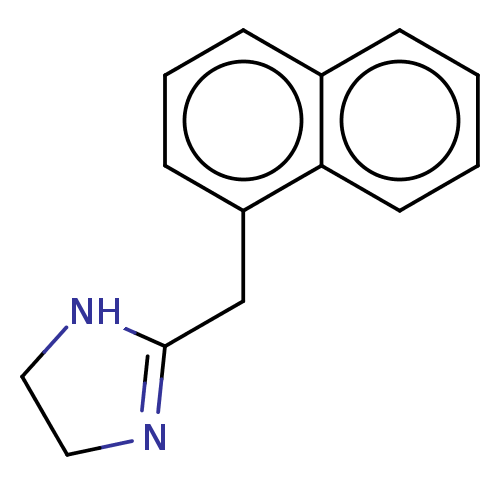 (2-Naphthalen-1-ylmethyl-4,5-dihydro-1H-imidazole; ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | n/a | n/a | 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230389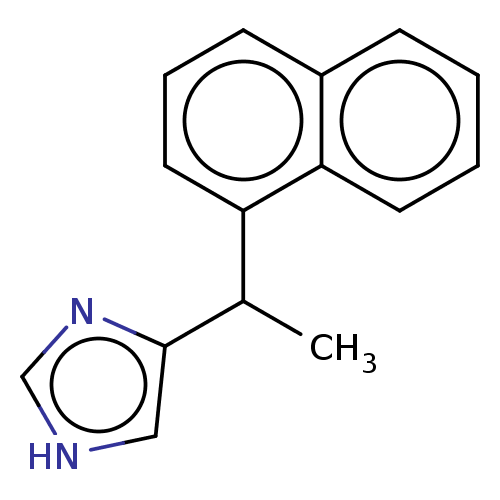 (CHEMBL541105) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was tested for Adrenergic activity against Alpha-2 adrenergic receptor from rat aorta | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230393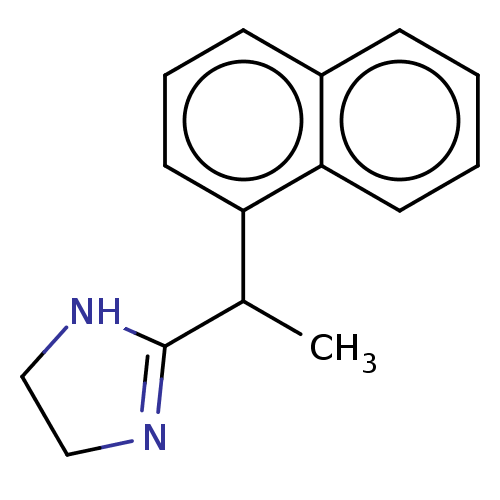 (CHEMBL553231) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230395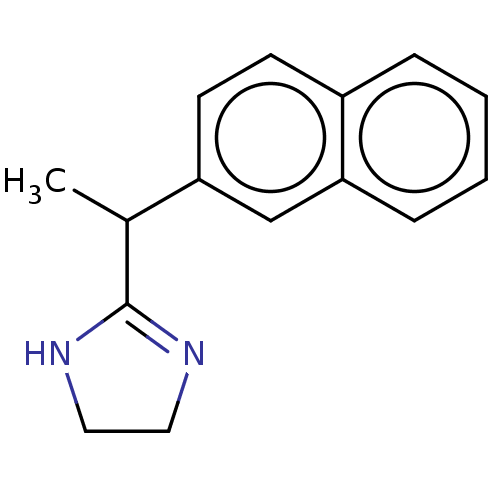 (CHEMBL557985) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230388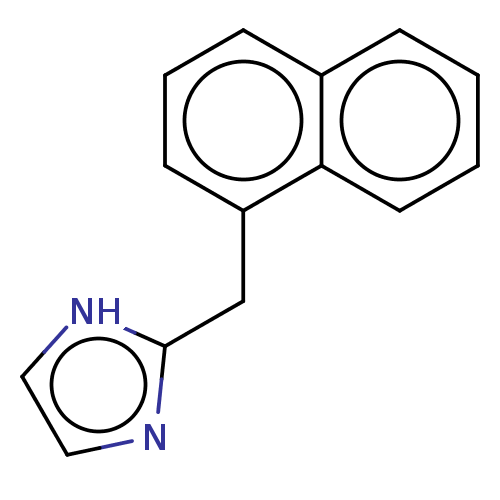 (CHEMBL537830) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230394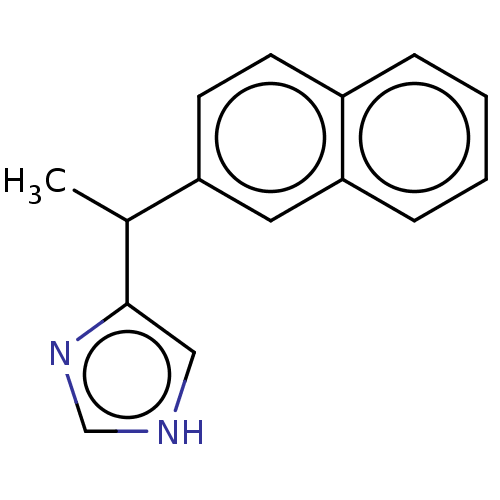 (CHEMBL538801) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230392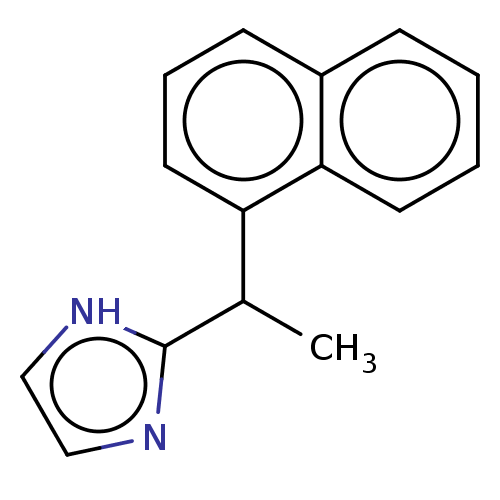 (CHEMBL554581) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50230391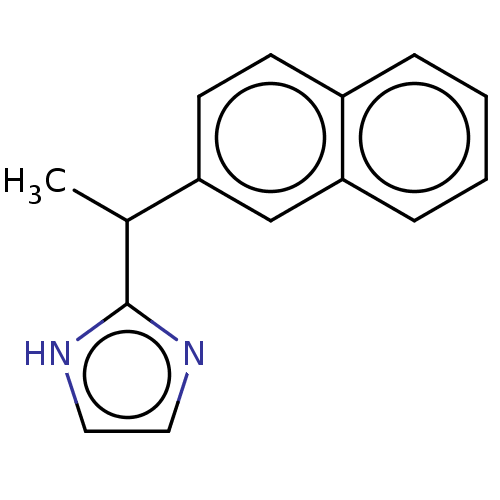 (CHEMBL164540) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was evaluated for Adrenergic activity against Alpha-2 adrenergic receptor in human platelets | J Med Chem 35: 750-5 (1992) BindingDB Entry DOI: 10.7270/Q27H1MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049128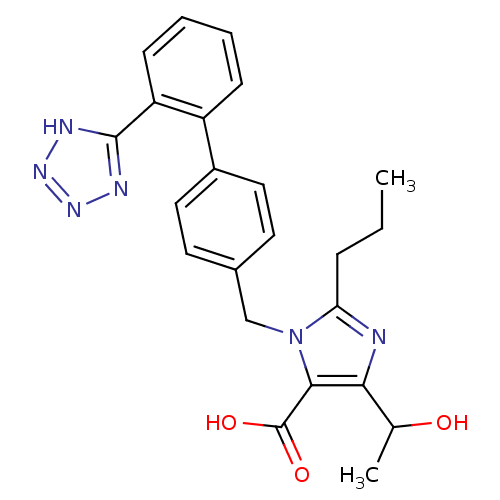 (5-(1-Hydroxy-ethyl)-2-propyl-3-[2'-(2H-tetrazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049118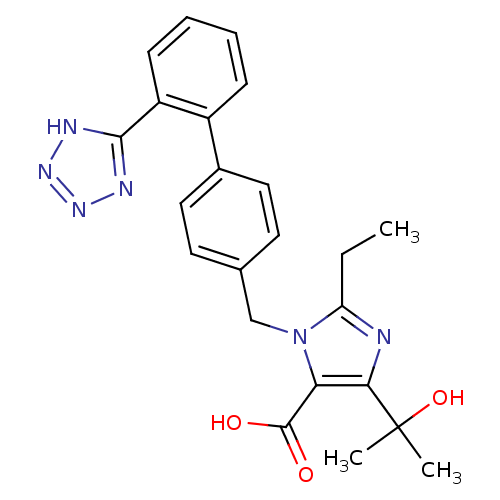 (2-Ethyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018179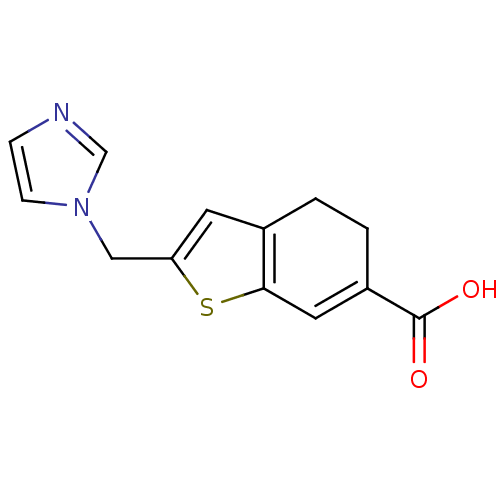 (2-Imidazol-1-ylmethyl-4,5-dihydro-benzo[b]thiophen...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of platelet microsomal thromboxane A synthetase in rabbits | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049123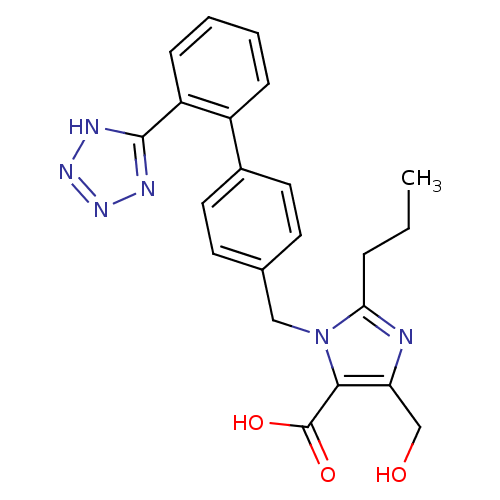 (5-Hydroxymethyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241364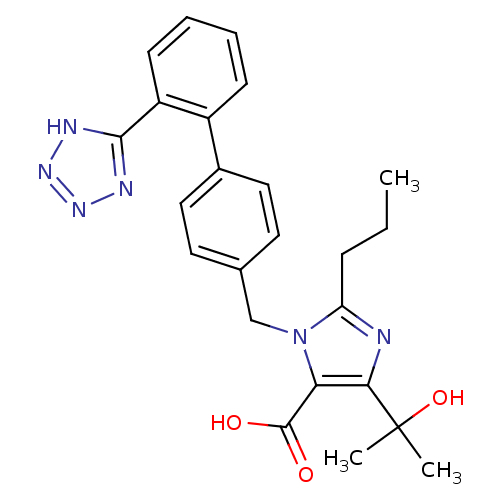 (4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(1H-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049107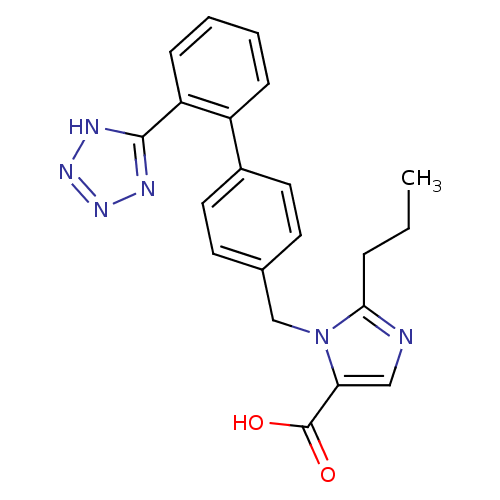 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049109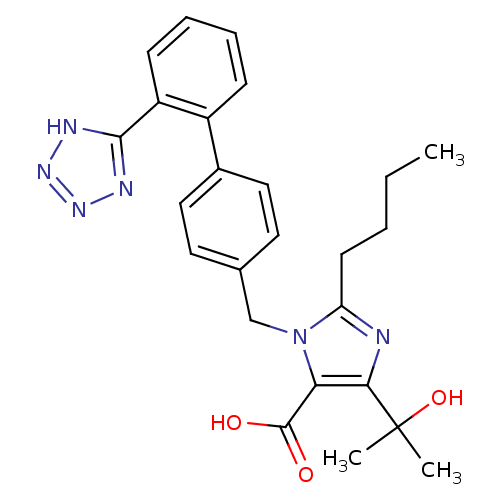 (2-Butyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049124 (5-Methyl-2-propyl-3-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049116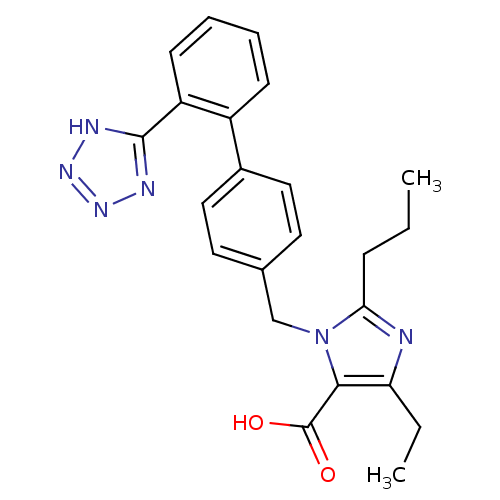 (5-Ethyl-2-propyl-3-(2'-tetrazol-1-yl-biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50018179 (2-Imidazol-1-ylmethyl-4,5-dihydro-benzo[b]thiophen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of platelet microsomal thromboxane A2 synthetase in rabbits | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018179 (2-Imidazol-1-ylmethyl-4,5-dihydro-benzo[b]thiophen...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018181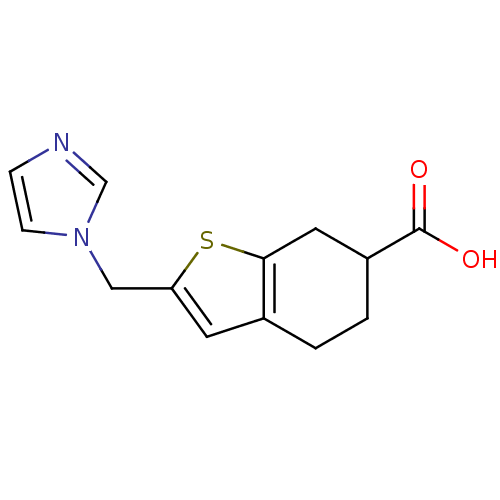 (2-Imidazol-1-ylmethyl-4,5,6,7-tetrahydro-benzo[b]t...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of platelet microsomal thromboxane A synthetase in humans | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50006909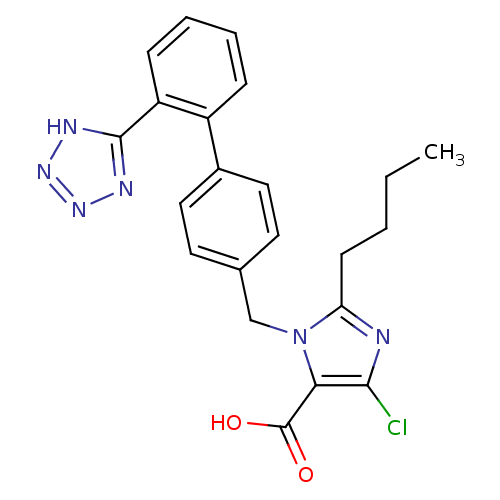 (2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50006909 (2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50018181 (2-Imidazol-1-ylmethyl-4,5,6,7-tetrahydro-benzo[b]t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018181 (2-Imidazol-1-ylmethyl-4,5,6,7-tetrahydro-benzo[b]t...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of platelet microsomal thromboxane A synthetase in humans | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049112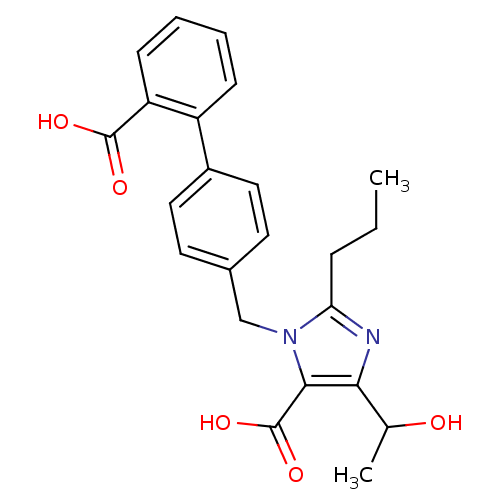 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049132 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049122 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-(1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049117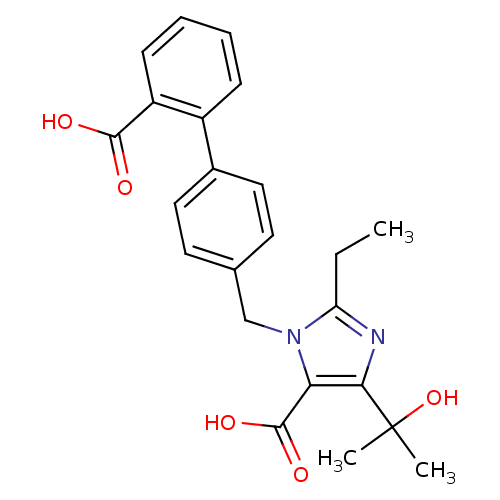 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-ethyl-5-(1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049130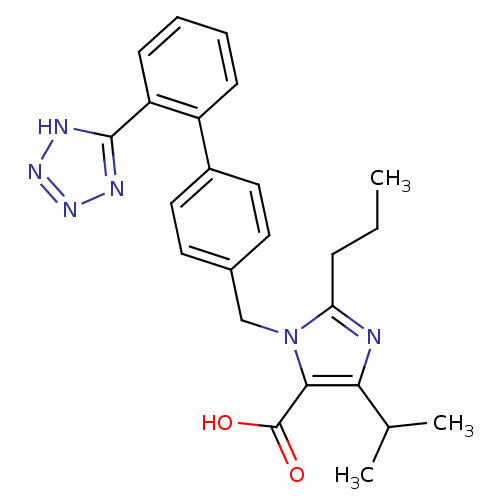 (5-Isopropyl-2-propyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049113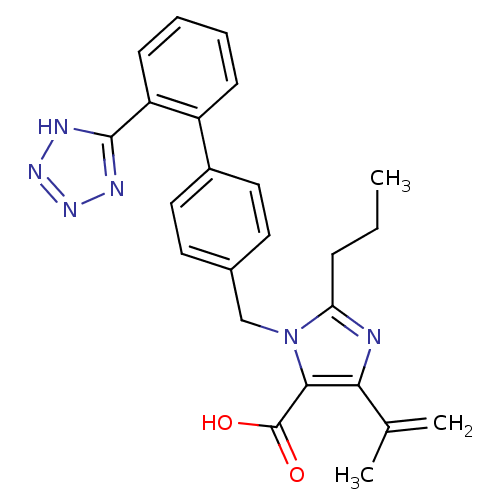 (5-Isopropenyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049106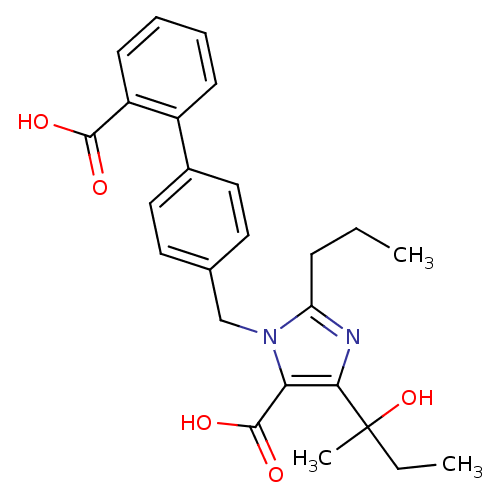 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049108 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049108 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049126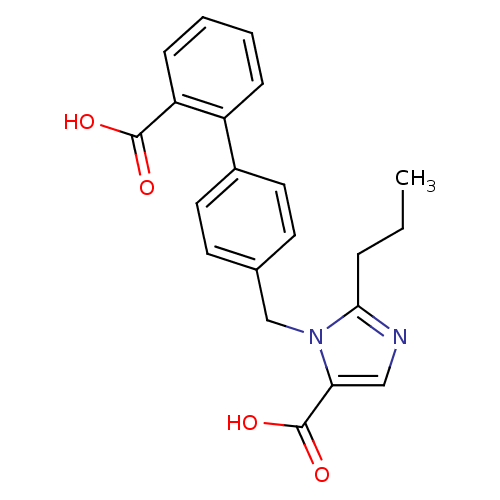 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-propyl-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282291 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018183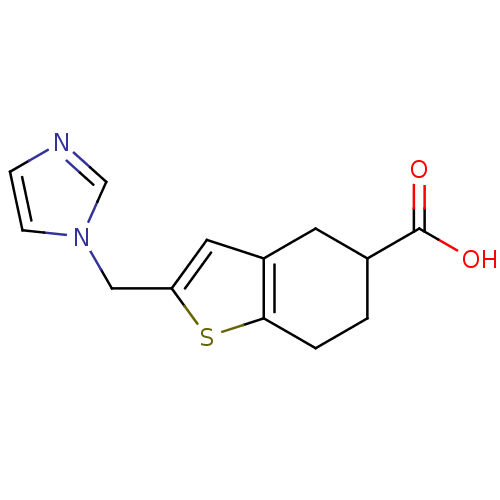 (2-Imidazol-1-ylmethyl-4,5,6,7-tetrahydro-benzo[b]t...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018186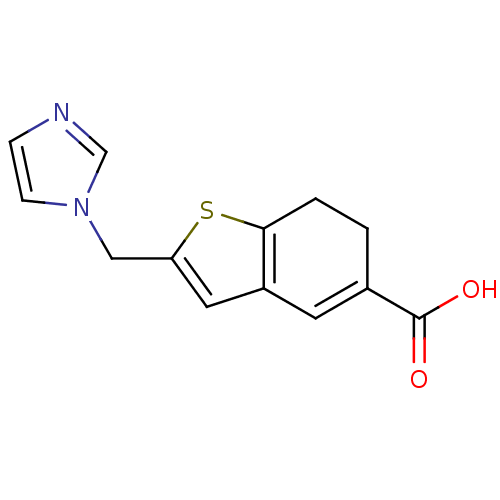 (2-Imidazol-1-ylmethyl-6,7-dihydro-benzo[b]thiophen...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049110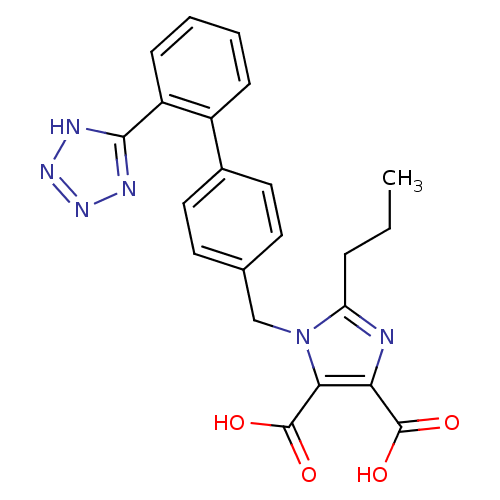 (2-Propyl-1-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049103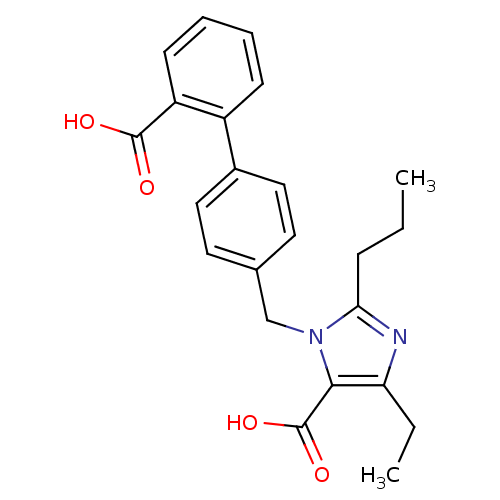 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-ethyl-2-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018178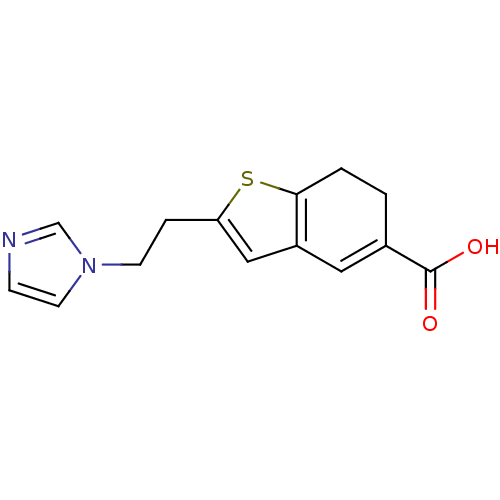 (2-(2-Imidazol-1-yl-ethyl)-6,7-dihydro-benzo[b]thio...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM7962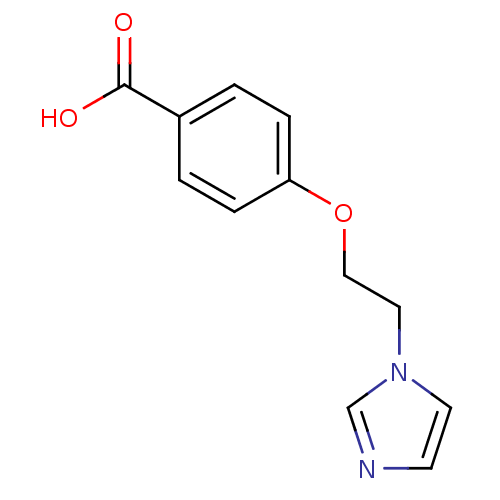 (4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM7962 (4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of platelet microsomal thromboxane A2 synthetase in rabbits | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049129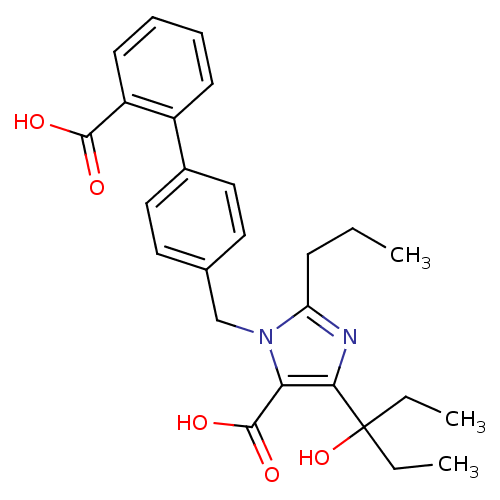 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-ethyl-1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049104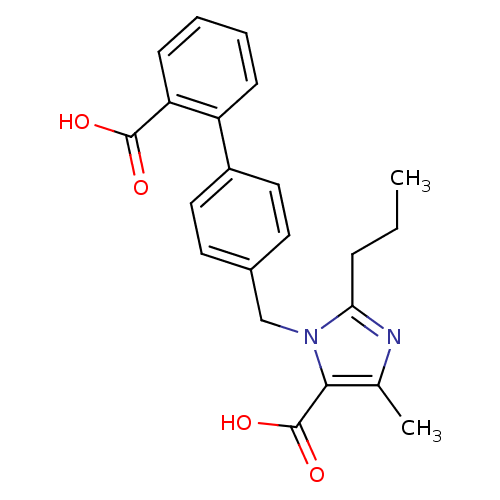 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-methyl-2-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018176 (2-(Imidazol-1-yl-phenyl-methyl)-6,7-dihydro-benzo[...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50018184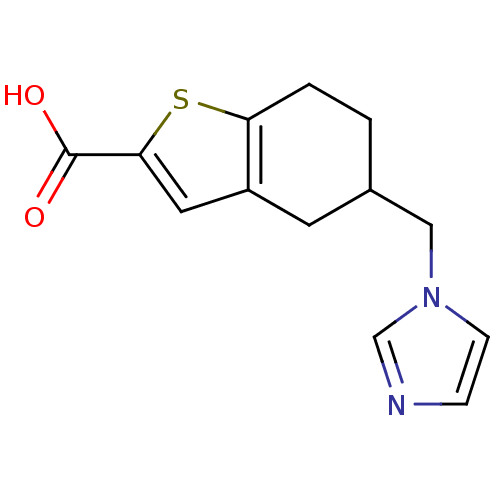 (5-Imidazol-1-ylmethyl-4,5,6,7-tetrahydro-benzo[b]t...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase | J Med Chem 32: 1265-72 (1989) BindingDB Entry DOI: 10.7270/Q2FF3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714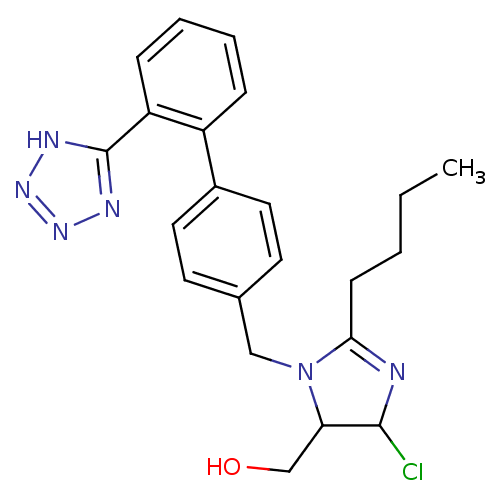 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009505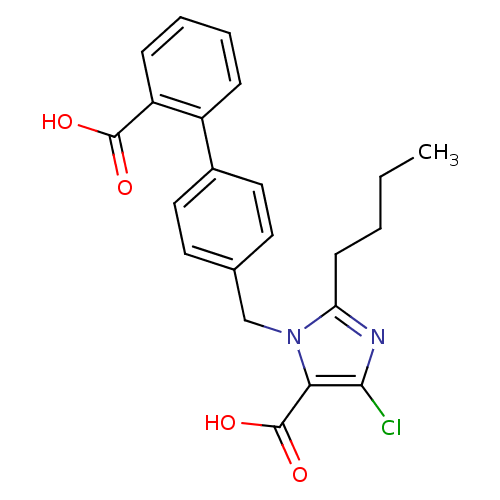 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009505 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |