 Found 489 hits with Last Name = 'arai' and Initial = 'y'
Found 489 hits with Last Name = 'arai' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227045
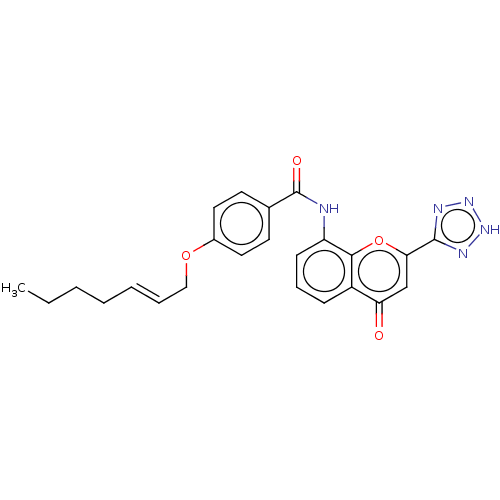
(CHEMBL354129)Show SMILES CCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C24H23N5O4/c1-2-3-4-5-6-14-32-17-12-10-16(11-13-17)24(31)25-19-9-7-8-18-20(30)15-21(33-22(18)19)23-26-28-29-27-23/h5-13,15H,2-4,14H2,1H3,(H,25,31)(H,26,27,28,29)/b6-5+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227075
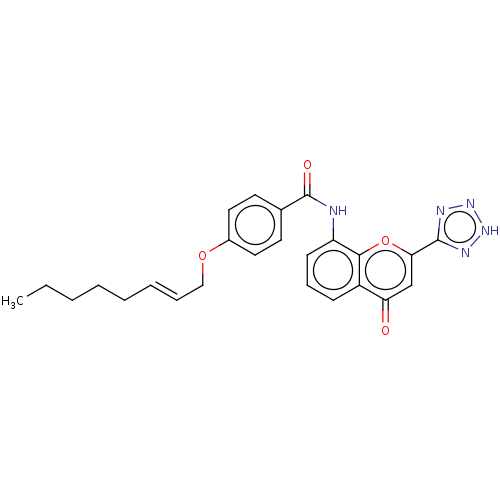
(CHEMBL171021)Show SMILES CCCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C25H25N5O4/c1-2-3-4-5-6-7-15-33-18-13-11-17(12-14-18)25(32)26-20-10-8-9-19-21(31)16-22(34-23(19)20)24-27-29-30-28-24/h6-14,16H,2-5,15H2,1H3,(H,26,32)(H,27,28,29,30)/b7-6+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50135039
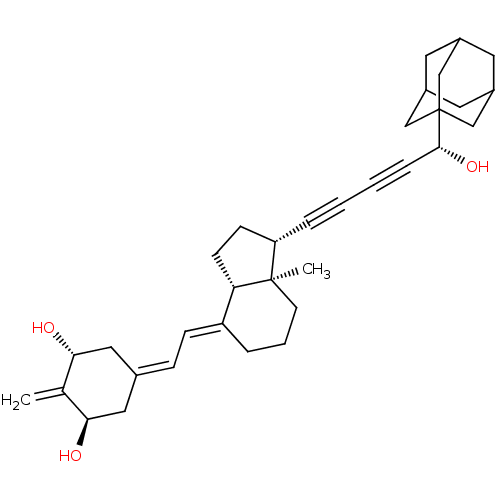
(CHEMBL3745849)Show SMILES [H][C@@]12[#6]-[#6]-[#6@H](C#CC#C[#6@@H](-[#8])C34[#6]-[#6]-5-[#6]-[#6](-[#6]-[#6](-[#6]-5)-[#6]3)-[#6]4)[C@@]1([#6])[#6]-[#6]-[#6]\[#6]2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C34H44O3/c1-22-30(35)17-23(18-31(22)36)9-10-27-6-5-13-33(2)28(11-12-29(27)33)7-3-4-8-32(37)34-19-24-14-25(20-34)16-26(15-24)21-34/h9-10,24-26,28-32,35-37H,1,5-6,11-21H2,2H3/b27-10+/t24?,25?,26?,28-,29-,30+,31+,32+,33+,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Agonist activity at VDR (unknown origin) expressed in HEK293 cells cotransfected with NCoR assessed as decrease in NCoR recruitment by two-hybrid ass... |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Agonist activity at VDR (unknown origin) expressed in HEK293 cells cotransfected with NCoR assessed as decrease in NCoR recruitment by two-hybrid ass... |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227072
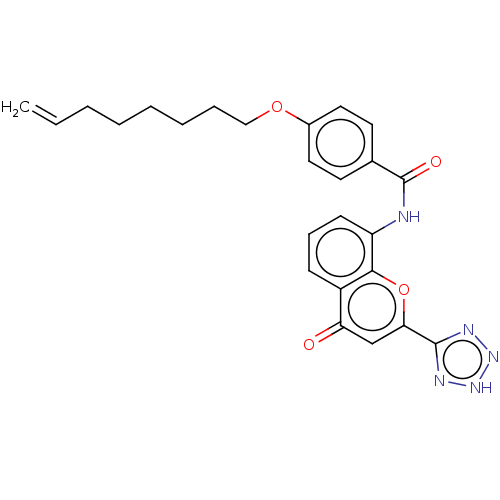
(CHEMBL169790)Show SMILES C=CCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C25H25N5O4/c1-2-3-4-5-6-7-15-33-18-13-11-17(12-14-18)25(32)26-20-10-8-9-19-21(31)16-22(34-23(19)20)24-27-29-30-28-24/h2,8-14,16H,1,3-7,15H2,(H,26,32)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227054
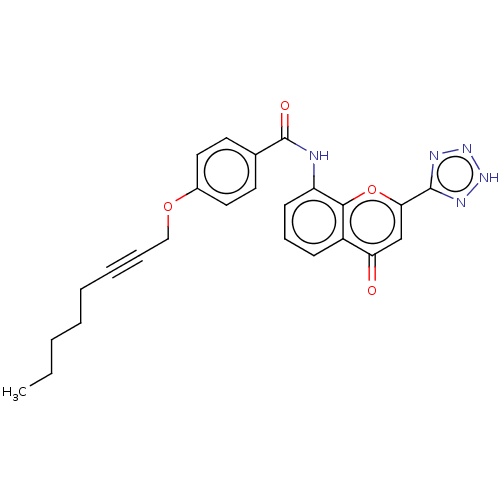
(CHEMBL354530)Show SMILES CCCCCC#CCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C25H23N5O4/c1-2-3-4-5-6-7-15-33-18-13-11-17(12-14-18)25(32)26-20-10-8-9-19-21(31)16-22(34-23(19)20)24-27-29-30-28-24/h8-14,16H,2-5,15H2,1H3,(H,26,32)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227053
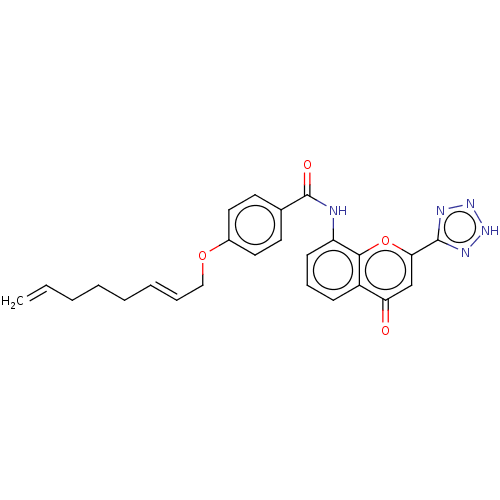
(CHEMBL170191)Show SMILES C=CCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C25H23N5O4/c1-2-3-4-5-6-7-15-33-18-13-11-17(12-14-18)25(32)26-20-10-8-9-19-21(31)16-22(34-23(19)20)24-27-29-30-28-24/h2,6-14,16H,1,3-5,15H2,(H,26,32)(H,27,28,29,30)/b7-6+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from recombinant human VDR ligand binding domain |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227047
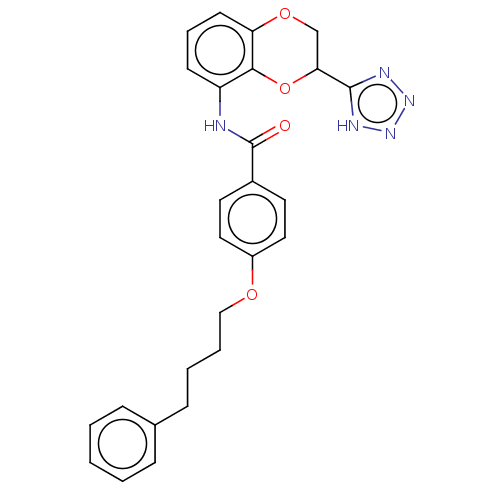
(CHEMBL172889)Show SMILES O=C(Nc1cccc2OCC(Oc12)c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C26H25N5O4/c32-26(19-12-14-20(15-13-19)33-16-5-4-9-18-7-2-1-3-8-18)27-21-10-6-11-22-24(21)35-23(17-34-22)25-28-30-31-29-25/h1-3,6-8,10-15,23H,4-5,9,16-17H2,(H,27,32)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227136
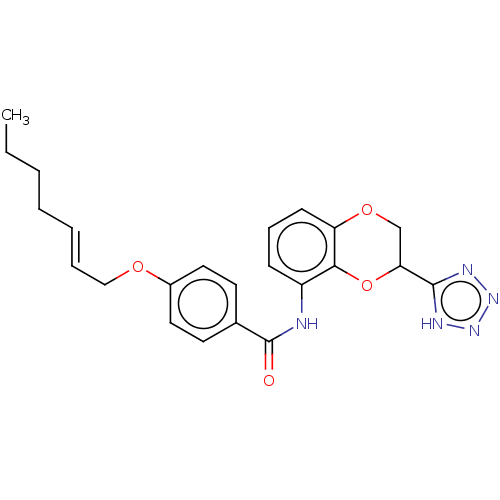
(CHEMBL170100)Show SMILES CCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C23H25N5O4/c1-2-3-4-5-6-14-30-17-12-10-16(11-13-17)23(29)24-18-8-7-9-19-21(18)32-20(15-31-19)22-25-27-28-26-22/h5-13,20H,2-4,14-15H2,1H3,(H,24,29)(H,25,26,27,28)/b6-5+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227144

(CHEMBL83797)Show SMILES CCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C24H25N5O4/c1-2-3-4-5-6-14-32-17-12-10-16(11-13-17)24(31)25-19-9-7-8-18-20(30)15-21(33-22(18)19)23-26-28-29-27-23/h7-13,15H,2-6,14H2,1H3,(H,25,31)(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50015318
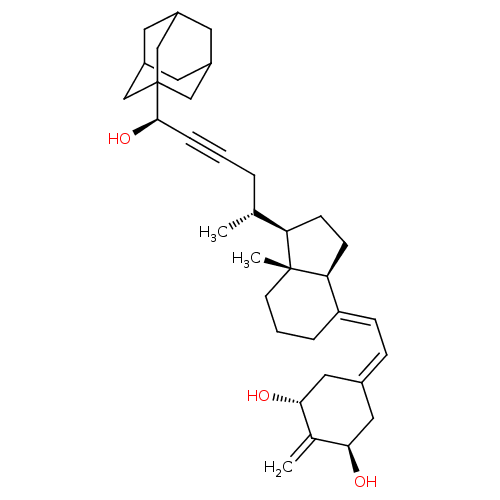
(CHEMBL3263871)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]C#C[#6@@H](-[#8])C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2 |r,THB:35:34:31:37.36.38,35:36:33.34.39:31,38:36:33:39.30.31,38:30:33:37.35.36| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-25-14-26(20-35)16-27(15-25)21-35)29-11-12-30-28(7-5-13-34(29,30)3)10-9-24-17-31(36)23(2)32(37)18-24/h9-10,22,25-27,29-33,36-38H,2,5-7,11-21H2,1,3H3/b28-10+/t22-,25?,26?,27?,29-,30+,31-,32-,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 |
J Med Chem 57: 4073-87 (2014)
Article DOI: 10.1021/jm401989c
BindingDB Entry DOI: 10.7270/Q2ZS2Z2G |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227063

(CHEMBL170017)Show SMILES CCCCCC#CCOc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C24H25N5O4/c1-2-3-4-5-6-7-15-31-18-13-11-17(12-14-18)24(30)25-19-9-8-10-20-22(19)33-21(16-32-20)23-26-28-29-27-23/h8-14,21H,2-5,15-16H2,1H3,(H,25,30)(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227040

(CHEMBL312105)Show SMILES CCCCCCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C23H23N5O4/c1-2-3-4-5-13-31-16-11-9-15(10-12-16)23(30)24-18-8-6-7-17-19(29)14-20(32-21(17)18)22-25-27-28-26-22/h6-12,14H,2-5,13H2,1H3,(H,24,30)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227140
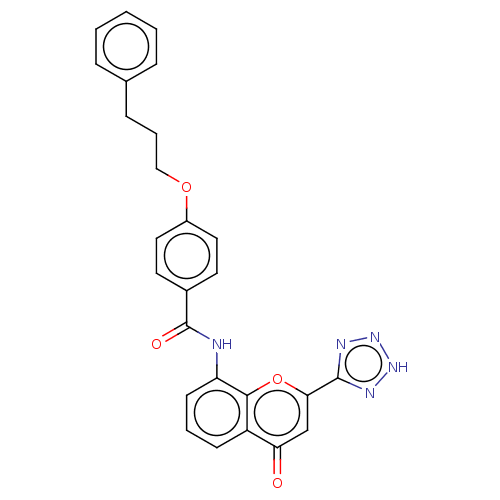
(CHEMBL171689)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1)c1ccc(OCCCc2ccccc2)cc1 Show InChI InChI=1S/C26H21N5O4/c32-22-16-23(25-28-30-31-29-25)35-24-20(22)9-4-10-21(24)27-26(33)18-11-13-19(14-12-18)34-15-5-8-17-6-2-1-3-7-17/h1-4,6-7,9-14,16H,5,8,15H2,(H,27,33)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227190

(CHEMBL171022)Show SMILES C=CCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C24H25N5O4/c1-2-3-4-5-6-7-15-31-18-13-11-17(12-14-18)24(30)25-19-9-8-10-20-22(19)33-21(16-32-20)23-26-28-29-27-23/h2,6-14,21H,1,3-5,15-16H2,(H,25,30)(H,26,27,28,29)/b7-6+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227060

(CHEMBL170959)Show SMILES C=CCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C24H27N5O4/c1-2-3-4-5-6-7-15-31-18-13-11-17(12-14-18)24(30)25-19-9-8-10-20-22(19)33-21(16-32-20)23-26-28-29-27-23/h2,8-14,21H,1,3-7,15-16H2,(H,25,30)(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50532176
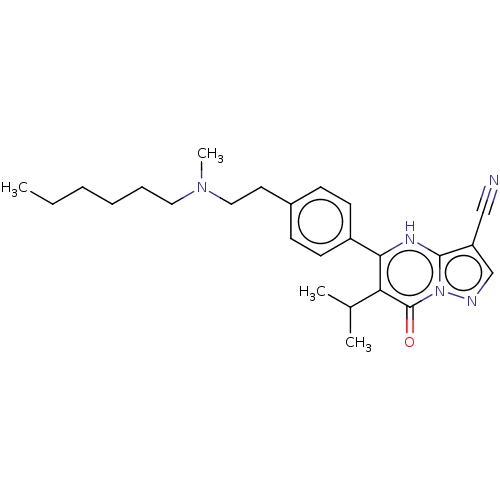
(CHEMBL4542928)Show SMILES CCCCCCN(C)CCc1ccc(cc1)-c1[nH]c2c(cnn2c(=O)c1C(C)C)C#N Show InChI InChI=1S/C25H33N5O/c1-5-6-7-8-14-29(4)15-13-19-9-11-20(12-10-19)23-22(18(2)3)25(31)30-24(28-23)21(16-26)17-27-30/h9-12,17-18,28H,5-8,13-15H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG/His-tagged KDM5B (2 to 751 residues) expressed in baculovirus infected Sf9 insect cells using H3(1-21)K4(Me3)-GGK... |
Bioorg Med Chem 27: 1119-1129 (2019)
Article DOI: 10.1016/j.bmc.2019.02.006
BindingDB Entry DOI: 10.7270/Q2668HPR |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227137
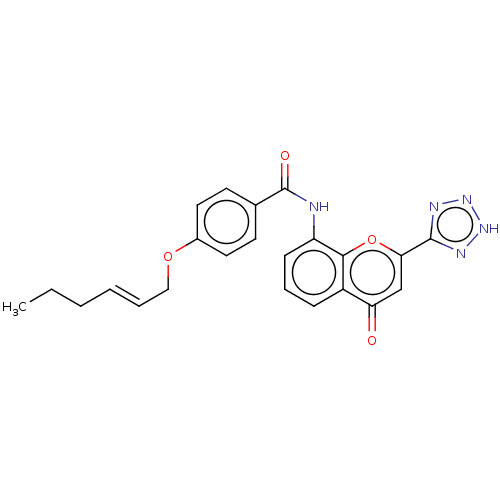
(CHEMBL422917)Show SMILES CCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C23H21N5O4/c1-2-3-4-5-13-31-16-11-9-15(10-12-16)23(30)24-18-8-6-7-17-19(29)14-20(32-21(17)18)22-25-27-28-26-22/h4-12,14H,2-3,13H2,1H3,(H,24,30)(H,25,26,27,28)/b5-4+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227086
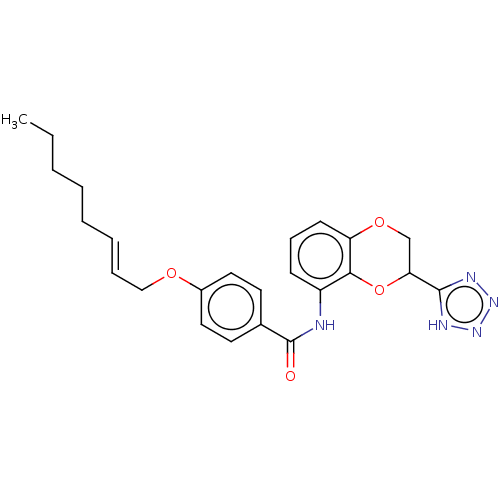
(CHEMBL170393)Show SMILES CCCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C24H27N5O4/c1-2-3-4-5-6-7-15-31-18-13-11-17(12-14-18)24(30)25-19-9-8-10-20-22(19)33-21(16-32-20)23-26-28-29-27-23/h6-14,21H,2-5,15-16H2,1H3,(H,25,30)(H,26,27,28,29)/b7-6+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50251742

((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...)Show SMILES CC(C)C[C@H](NP(O)(=O)O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C23H34N3O10P/c1-11(2)8-16(26-37(33,34)36-23-20(29)19(28)18(27)12(3)35-23)21(30)25-17(22(31)32)9-13-10-24-15-7-5-4-6-14(13)15/h4-7,10-12,16-20,23-24,27-29H,8-9H2,1-3H3,(H,25,30)(H,31,32)(H2,26,33,34)/t12-,16-,17-,18-,19+,20+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine |
Bioorg Med Chem Lett 4: 1257-1262 (1994)
Article DOI: 10.1016/S0960-894X(01)80341-3
BindingDB Entry DOI: 10.7270/Q2KS6S2H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227051
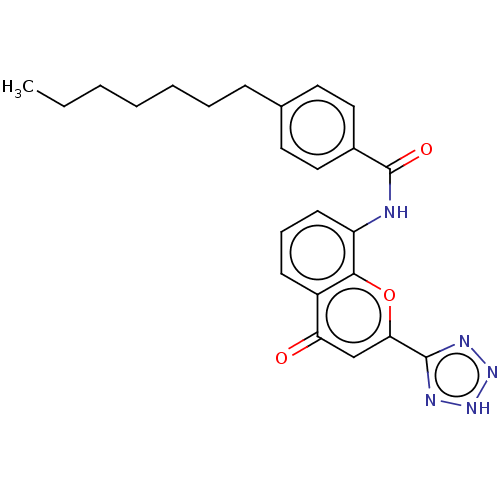
(CHEMBL81565)Show SMILES CCCCCCCc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C24H25N5O3/c1-2-3-4-5-6-8-16-11-13-17(14-12-16)24(31)25-19-10-7-9-18-20(30)15-21(32-22(18)19)23-26-28-29-27-23/h7,9-15H,2-6,8H2,1H3,(H,25,31)(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227041
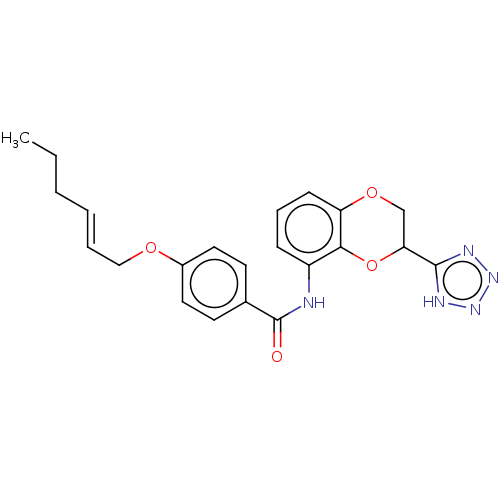
(CHEMBL353429)Show SMILES CCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C22H23N5O4/c1-2-3-4-5-13-29-16-11-9-15(10-12-16)22(28)23-17-7-6-8-18-20(17)31-19(14-30-18)21-24-26-27-25-21/h4-12,19H,2-3,13-14H2,1H3,(H,23,28)(H,24,25,26,27)/b5-4+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50532176

(CHEMBL4542928)Show SMILES CCCCCCN(C)CCc1ccc(cc1)-c1[nH]c2c(cnn2c(=O)c1C(C)C)C#N Show InChI InChI=1S/C25H33N5O/c1-5-6-7-8-14-29(4)15-13-19-9-11-20(12-10-19)23-22(18(2)3)25(31)30-24(28-23)21(16-26)17-27-30/h9-12,17-18,28H,5-8,13-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal FLAG-tagged KDM5A (1 to 1090 residues) expressed in baculovirus infected Sf9 insect cells using H3(1-21)K4(Me3)-GGK(Bi... |
Bioorg Med Chem 27: 1119-1129 (2019)
Article DOI: 10.1016/j.bmc.2019.02.006
BindingDB Entry DOI: 10.7270/Q2668HPR |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50135039

(CHEMBL3745849)Show SMILES [H][C@@]12[#6]-[#6]-[#6@H](C#CC#C[#6@@H](-[#8])C34[#6]-[#6]-5-[#6]-[#6](-[#6]-[#6](-[#6]-5)-[#6]3)-[#6]4)[C@@]1([#6])[#6]-[#6]-[#6]\[#6]2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C34H44O3/c1-22-30(35)17-23(18-31(22)36)9-10-27-6-5-13-33(2)28(11-12-29(27)33)7-3-4-8-32(37)34-19-24-14-25(20-34)16-26(15-24)21-34/h9-10,24-26,28-32,35-37H,1,5-6,11-21H2,2H3/b27-10+/t24?,25?,26?,28-,29-,30+,31+,32+,33+,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from recombinant human VDR ligand binding domain |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227064
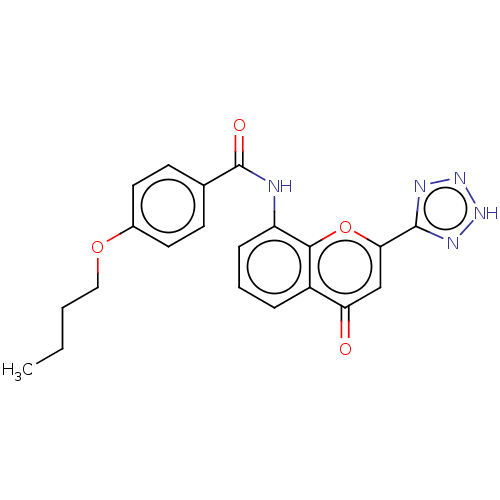
(CHEMBL172766)Show SMILES CCCCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C21H19N5O4/c1-2-3-11-29-14-9-7-13(8-10-14)21(28)22-16-6-4-5-15-17(27)12-18(30-19(15)16)20-23-25-26-24-20/h4-10,12H,2-3,11H2,1H3,(H,22,28)(H,23,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227098
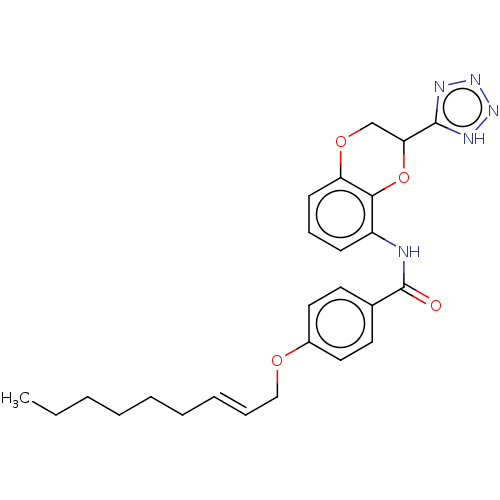
(CHEMBL170506)Show SMILES CCCCCC\C=C\COc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C25H29N5O4/c1-2-3-4-5-6-7-8-16-32-19-14-12-18(13-15-19)25(31)26-20-10-9-11-21-23(20)34-22(17-33-21)24-27-29-30-28-24/h7-15,22H,2-6,16-17H2,1H3,(H,26,31)(H,27,28,29,30)/b8-7+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227033
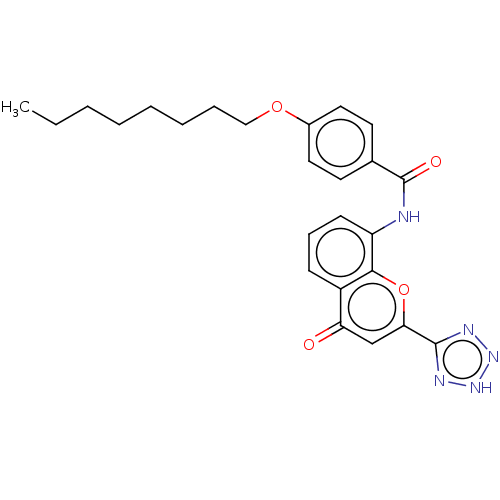
(CHEMBL311891)Show SMILES CCCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C25H27N5O4/c1-2-3-4-5-6-7-15-33-18-13-11-17(12-14-18)25(32)26-20-10-8-9-19-21(31)16-22(34-23(19)20)24-27-29-30-28-24/h8-14,16H,2-7,15H2,1H3,(H,26,32)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227079
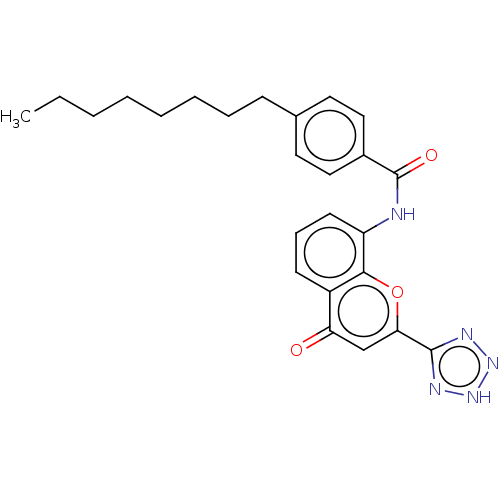
(CHEMBL83447)Show SMILES CCCCCCCCc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C25H27N5O3/c1-2-3-4-5-6-7-9-17-12-14-18(15-13-17)25(32)26-20-11-8-10-19-21(31)16-22(33-23(19)20)24-27-29-30-28-24/h8,10-16H,2-7,9H2,1H3,(H,26,32)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM50532173
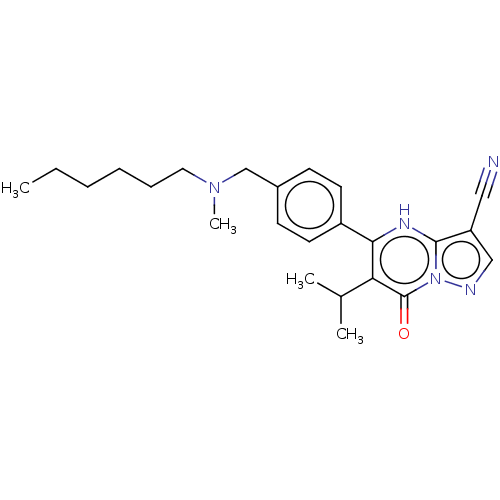
(CHEMBL4536443)Show SMILES CCCCCCN(C)Cc1ccc(cc1)-c1[nH]c2c(cnn2c(=O)c1C(C)C)C#N Show InChI InChI=1S/C24H31N5O/c1-5-6-7-8-13-28(4)16-18-9-11-19(12-10-18)22-21(17(2)3)24(30)29-23(27-22)20(14-25)15-26-29/h9-12,15,17,27H,5-8,13,16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal FLAG/His-tagged KDM5C (2 to 1560 residues) expressed in baculovirus infected Sf9 insect cells using H3(1-2... |
Bioorg Med Chem 27: 1119-1129 (2019)
Article DOI: 10.1016/j.bmc.2019.02.006
BindingDB Entry DOI: 10.7270/Q2668HPR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM50532176

(CHEMBL4542928)Show SMILES CCCCCCN(C)CCc1ccc(cc1)-c1[nH]c2c(cnn2c(=O)c1C(C)C)C#N Show InChI InChI=1S/C25H33N5O/c1-5-6-7-8-14-29(4)15-13-19-9-11-20(12-10-19)23-22(18(2)3)25(31)30-24(28-23)21(16-26)17-27-30/h9-12,17-18,28H,5-8,13-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal FLAG/His-tagged KDM5C (2 to 1560 residues) expressed in baculovirus infected Sf9 insect cells using H3(1-2... |
Bioorg Med Chem 27: 1119-1129 (2019)
Article DOI: 10.1016/j.bmc.2019.02.006
BindingDB Entry DOI: 10.7270/Q2668HPR |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227074
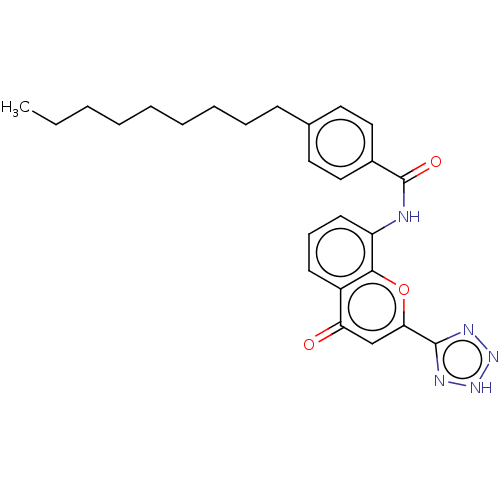
(CHEMBL312051)Show SMILES CCCCCCCCCc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C26H29N5O3/c1-2-3-4-5-6-7-8-10-18-13-15-19(16-14-18)26(33)27-21-12-9-11-20-22(32)17-23(34-24(20)21)25-28-30-31-29-25/h9,11-17H,2-8,10H2,1H3,(H,27,33)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227036

(CHEMBL312090)Show SMILES CCCCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C26H29N5O4/c1-2-3-4-5-6-7-8-16-34-19-14-12-18(13-15-19)26(33)27-21-11-9-10-20-22(32)17-23(35-24(20)21)25-28-30-31-29-25/h9-15,17H,2-8,16H2,1H3,(H,27,33)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227073
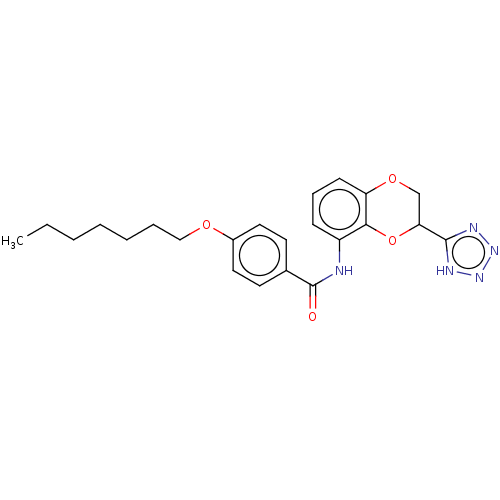
(CHEMBL80993)Show SMILES CCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C23H27N5O4/c1-2-3-4-5-6-14-30-17-12-10-16(11-13-17)23(29)24-18-8-7-9-19-21(18)32-20(15-31-19)22-25-27-28-26-22/h7-13,20H,2-6,14-15H2,1H3,(H,24,29)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50161394
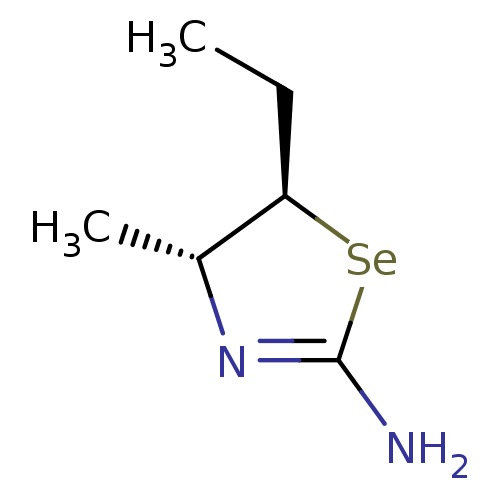
((4R,5R)-5-Ethyl-4-methyl-selenazolidin-(2Z)-yliden...)Show InChI InChI=1S/C6H12N2Se/c1-3-5-4(2)8-6(7)9-5/h4-5H,3H2,1-2H3,(H2,7,8)/t4-,5-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against inducible nitric acid synthase |
Bioorg Med Chem Lett 15: 1361-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.013
BindingDB Entry DOI: 10.7270/Q2G160B2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50161393
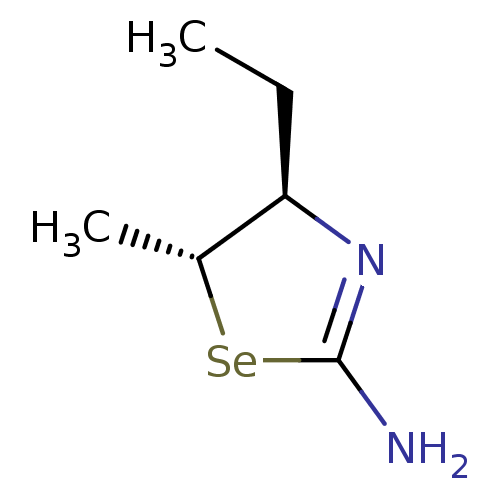
((4R,5R)-4-Ethyl-5-methyl-selenazolidin-(2Z)-yliden...)Show InChI InChI=1S/C6H12N2Se/c1-3-5-4(2)9-6(7)8-5/h4-5H,3H2,1-2H3,(H2,7,8)/t4-,5-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against inducible nitric acid synthase |
Bioorg Med Chem Lett 15: 1361-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.013
BindingDB Entry DOI: 10.7270/Q2G160B2 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227062

(CHEMBL174377)Show SMILES O=C(Nc1cccc2OCC(Oc12)c1nnn[nH]1)c1ccc(OCCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H27N5O4/c33-27(28-22-11-7-12-23-25(22)36-24(18-35-23)26-29-31-32-30-26)20-13-15-21(16-14-20)34-17-6-2-5-10-19-8-3-1-4-9-19/h1,3-4,7-9,11-16,24H,2,5-6,10,17-18H2,(H,28,33)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50015315
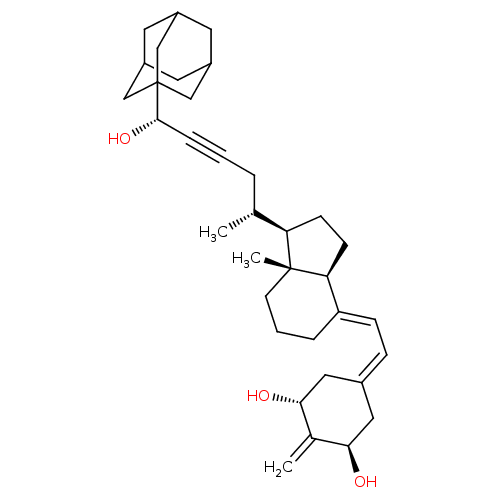
(CHEMBL3263870)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]C#C[#6@H](-[#8])C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2 |r,THB:35:34:31:37.36.38,35:36:33.34.39:31,38:36:33:39.30.31,38:30:33:37.35.36| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-25-14-26(20-35)16-27(15-25)21-35)29-11-12-30-28(7-5-13-34(29,30)3)10-9-24-17-31(36)23(2)32(37)18-24/h9-10,22,25-27,29-33,36-38H,2,5-7,11-21H2,1,3H3/b28-10+/t22-,25?,26?,27?,29-,30+,31-,32-,33+,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 |
J Med Chem 57: 4073-87 (2014)
Article DOI: 10.1021/jm401989c
BindingDB Entry DOI: 10.7270/Q2ZS2Z2G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50015316
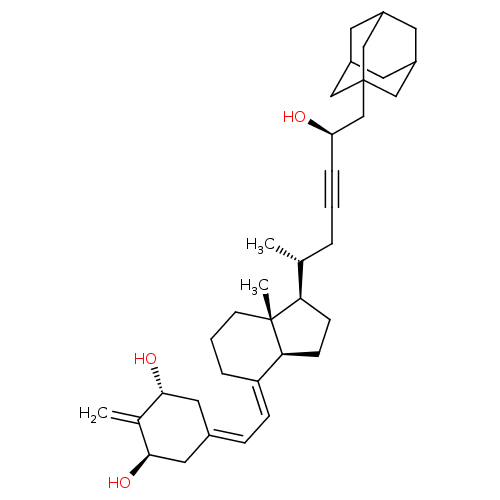
(CHEMBL3263873)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]C#C[#6@@H](-[#8])-[#6]C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2 |r,THB:36:35:32:38.37.39,36:37:34.35.40:32,39:37:34:40.31.32,39:31:34:38.36.37,30:31:34:38.36.37| Show InChI InChI=1S/C36H52O3/c1-23(6-4-8-30(37)22-36-19-26-14-27(20-36)16-28(15-26)21-36)31-11-12-32-29(7-5-13-35(31,32)3)10-9-25-17-33(38)24(2)34(39)18-25/h9-10,23,26-28,30-34,37-39H,2,5-7,11-22H2,1,3H3/b29-10+/t23-,26?,27?,28?,30-,31-,32+,33-,34-,35-,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 |
J Med Chem 57: 4073-87 (2014)
Article DOI: 10.1021/jm401989c
BindingDB Entry DOI: 10.7270/Q2ZS2Z2G |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227065
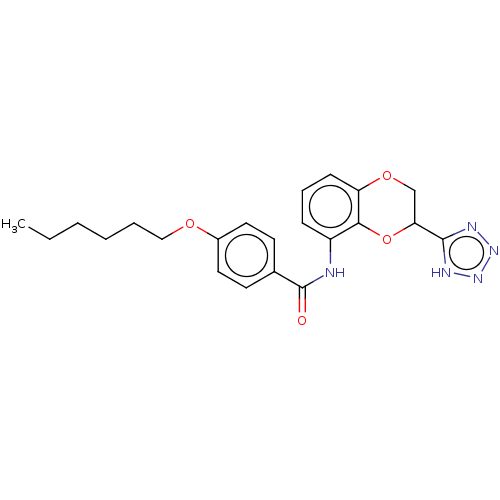
(CHEMBL82033)Show SMILES CCCCCCOc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C22H25N5O4/c1-2-3-4-5-13-29-16-11-9-15(10-12-16)22(28)23-17-7-6-8-18-20(17)31-19(14-30-18)21-24-26-27-25-21/h6-12,19H,2-5,13-14H2,1H3,(H,23,28)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50015317

(CHEMBL3263872)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]C#C[#6@H](-[#8])-[#6]C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2 |r,THB:36:35:32:38.37.39,36:37:34.35.40:32,39:37:34:40.31.32,39:31:34:38.36.37,30:31:34:38.36.37| Show InChI InChI=1S/C36H52O3/c1-23(6-4-8-30(37)22-36-19-26-14-27(20-36)16-28(15-26)21-36)31-11-12-32-29(7-5-13-35(31,32)3)10-9-25-17-33(38)24(2)34(39)18-25/h9-10,23,26-28,30-34,37-39H,2,5-7,11-22H2,1,3H3/b29-10+/t23-,26?,27?,28?,30+,31-,32+,33-,34-,35-,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 |
J Med Chem 57: 4073-87 (2014)
Article DOI: 10.1021/jm401989c
BindingDB Entry DOI: 10.7270/Q2ZS2Z2G |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50135031

(CHEMBL3747243)Show SMILES [H][C@@]12[#6]-[#6]-[#6@H](C#CC#C[#6@H](-[#8])C34[#6]-[#6]-5-[#6]-[#6](-[#6]-[#6](-[#6]-5)-[#6]3)-[#6]4)[C@@]1([#6])[#6]-[#6]-[#6]\[#6]2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C34H44O3/c1-22-30(35)17-23(18-31(22)36)9-10-27-6-5-13-33(2)28(11-12-29(27)33)7-3-4-8-32(37)34-19-24-14-25(20-34)16-26(15-24)21-34/h9-10,24-26,28-32,35-37H,1,5-6,11-21H2,2H3/b27-10+/t24?,25?,26?,28-,29-,30+,31+,32-,33+,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from recombinant human VDR ligand binding domain |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227035
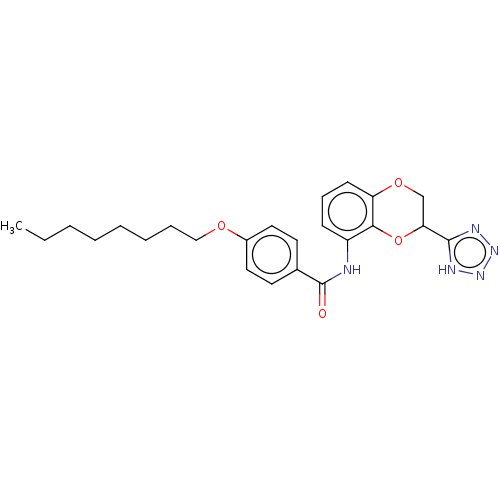
(CHEMBL311759)Show SMILES CCCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C24H29N5O4/c1-2-3-4-5-6-7-15-31-18-13-11-17(12-14-18)24(30)25-19-9-8-10-20-22(19)33-21(16-32-20)23-26-28-29-27-23/h8-14,21H,2-7,15-16H2,1H3,(H,25,30)(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227071
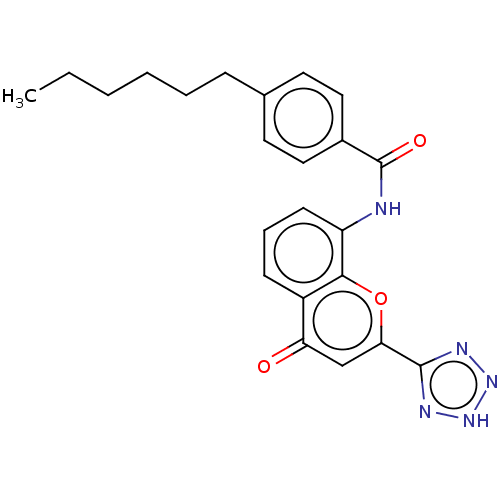
(CHEMBL78974)Show SMILES CCCCCCc1ccc(cc1)C(=O)Nc1cccc2c1oc(cc2=O)-c1nn[nH]n1 Show InChI InChI=1S/C23H23N5O3/c1-2-3-4-5-7-15-10-12-16(13-11-15)23(30)24-18-9-6-8-17-19(29)14-20(31-21(17)18)22-25-27-28-26-22/h6,8-14H,2-5,7H2,1H3,(H,24,30)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227188
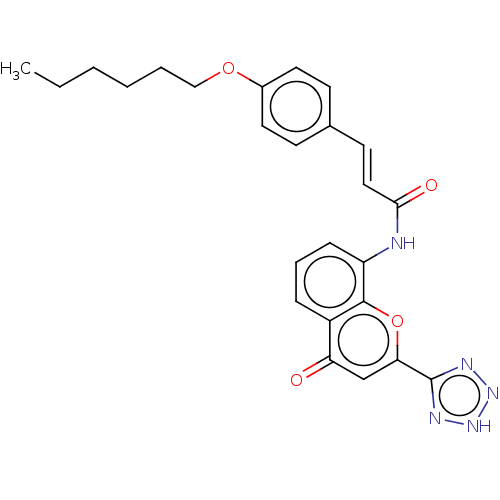
(CHEMBL171746)Show SMILES CCCCCCOc1ccc(\C=C\C(=O)Nc2cccc3c2oc(cc3=O)-c2nn[nH]n2)cc1 Show InChI InChI=1S/C25H25N5O4/c1-2-3-4-5-15-33-18-12-9-17(10-13-18)11-14-23(32)26-20-8-6-7-19-21(31)16-22(34-24(19)20)25-27-29-30-28-25/h6-14,16H,2-5,15H2,1H3,(H,26,32)(H,27,28,29,30)/b14-11+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227139
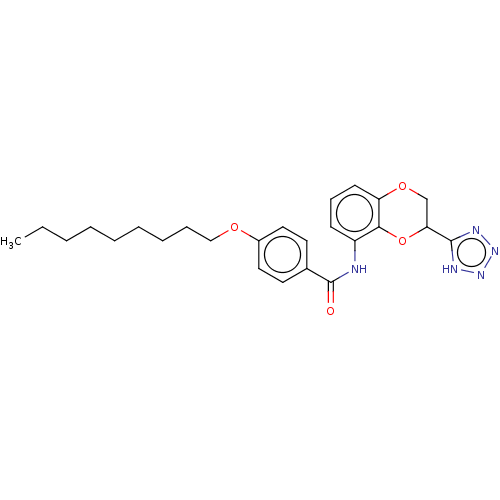
(CHEMBL81585)Show SMILES CCCCCCCCCOc1ccc(cc1)C(=O)Nc1cccc2OCC(Oc12)c1nnn[nH]1 Show InChI InChI=1S/C25H31N5O4/c1-2-3-4-5-6-7-8-16-32-19-14-12-18(13-15-19)25(31)26-20-10-9-11-21-23(20)34-22(17-33-21)24-27-29-30-28-24/h9-15,22H,2-8,16-17H2,1H3,(H,26,31)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227085
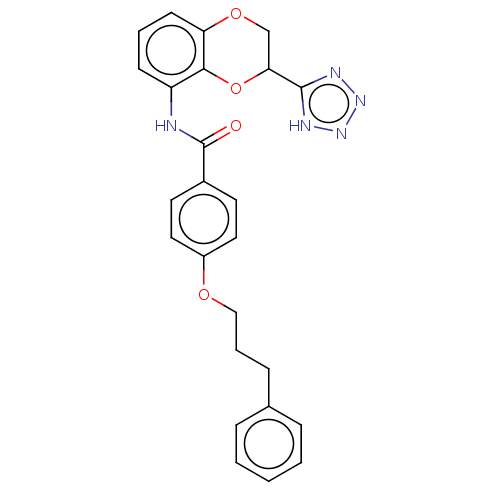
(CHEMBL173750)Show SMILES O=C(Nc1cccc2OCC(Oc12)c1nnn[nH]1)c1ccc(OCCCc2ccccc2)cc1 Show InChI InChI=1S/C25H23N5O4/c31-25(18-11-13-19(14-12-18)32-15-5-8-17-6-2-1-3-7-17)26-20-9-4-10-21-23(20)34-22(16-33-21)24-27-29-30-28-24/h1-4,6-7,9-14,22H,5,8,15-16H2,(H,26,31)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227084
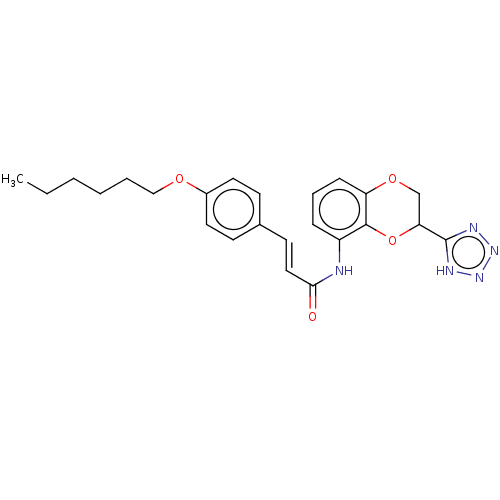
(CHEMBL354606)Show SMILES CCCCCCOc1ccc(\C=C\C(=O)Nc2cccc3OCC(Oc23)c2nnn[nH]2)cc1 Show InChI InChI=1S/C24H27N5O4/c1-2-3-4-5-15-31-18-12-9-17(10-13-18)11-14-22(30)25-19-7-6-8-20-23(19)33-21(16-32-20)24-26-28-29-27-24/h6-14,21H,2-5,15-16H2,1H3,(H,25,30)(H,26,27,28,29)/b14-11+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 induced smooth muscle contraction of guinea pig ileum |
J Med Chem 31: 84-91 (1988)
BindingDB Entry DOI: 10.7270/Q20K2BSP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50161389
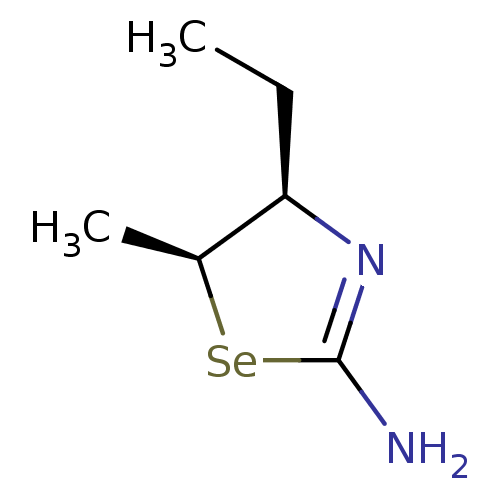
((4R,5S)-4-Ethyl-5-methyl-selenazolidin-(2Z)-yliden...)Show InChI InChI=1S/C6H12N2Se/c1-3-5-4(2)9-6(7)8-5/h4-5H,3H2,1-2H3,(H2,7,8)/t4-,5+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against inducible nitric acid synthase |
Bioorg Med Chem Lett 15: 1361-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.013
BindingDB Entry DOI: 10.7270/Q2G160B2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data