

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483937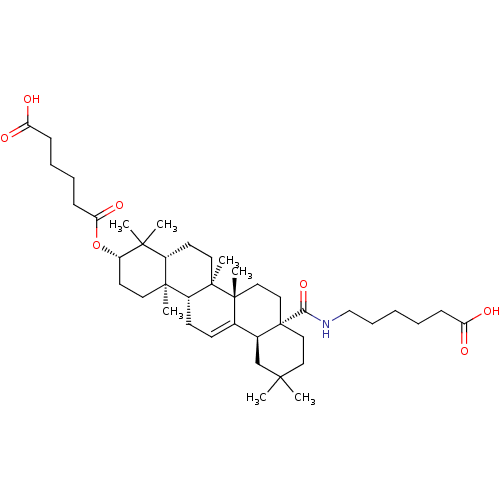 (CHEMBL1783813) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483935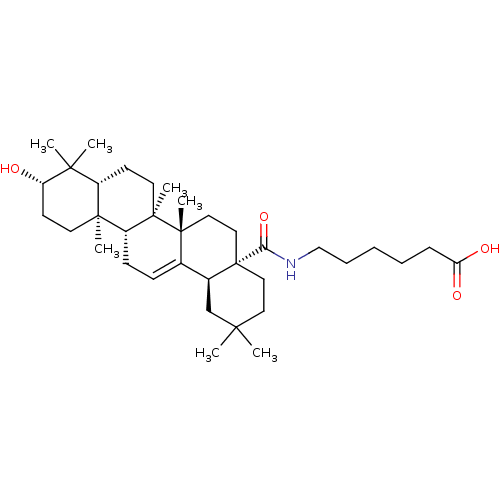 (CHEMBL1783812) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50315537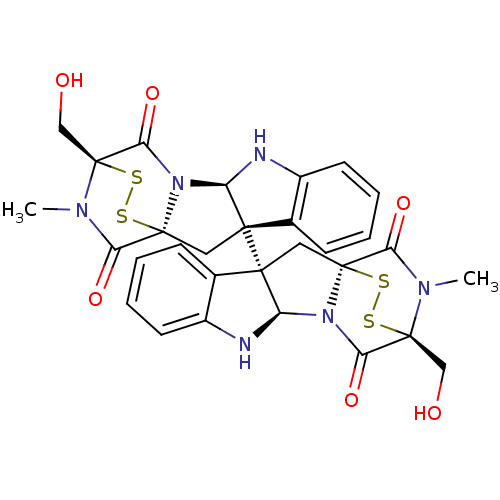 (CHEMBL1089316 | chaetocin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483932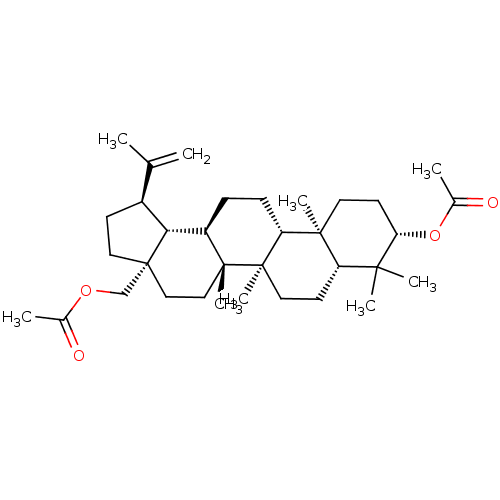 (Betulin 3,28-Diacetate | Betulin Diacetate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483934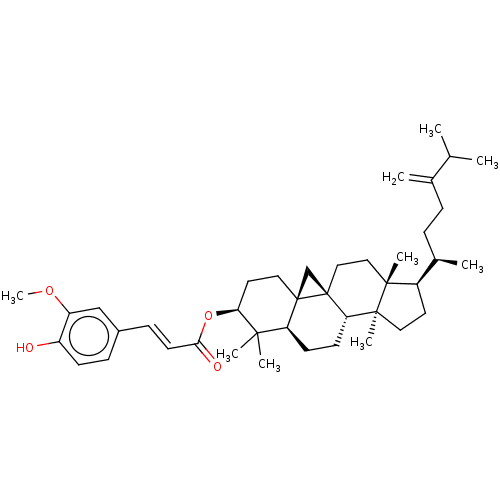 (24-Methylenecycloartanol Ferulate | 24-Methylenecy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425308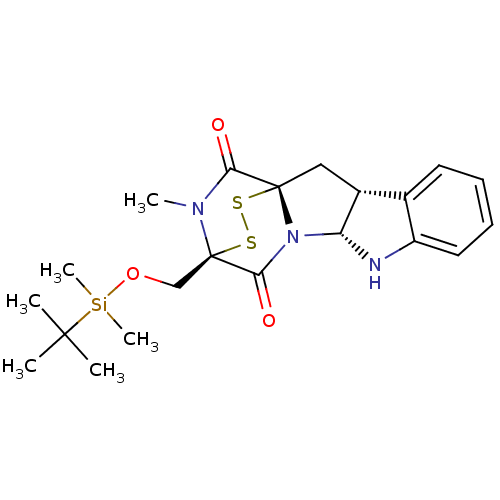 (CHEMBL2311579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483936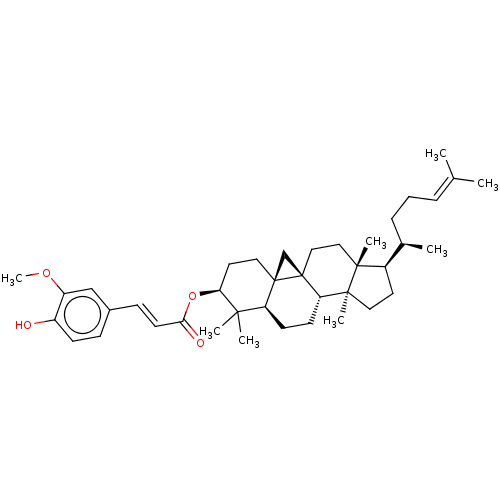 (3-O-Ferulylcycloartenol | Cycloartenol Ferulate | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM23208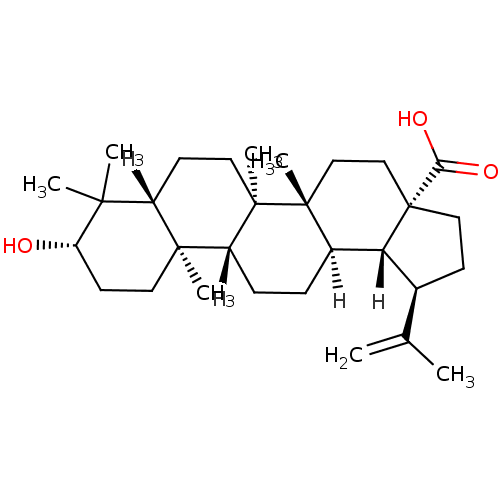 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-17-hydroxy-1,...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483933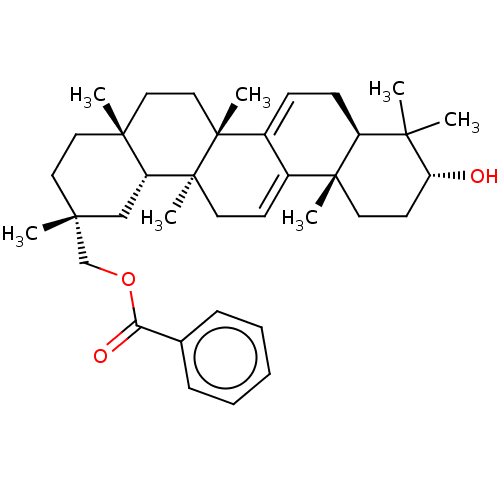 (CHEMBL1783816 | Karounidiol 29-Benzoate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50241944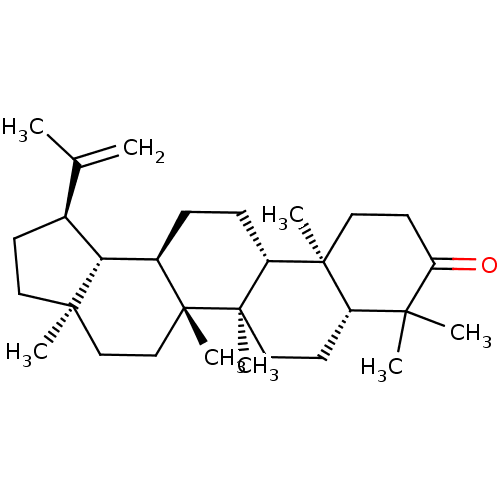 (CHEMBL486393 | Lupenone | lupeone) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425305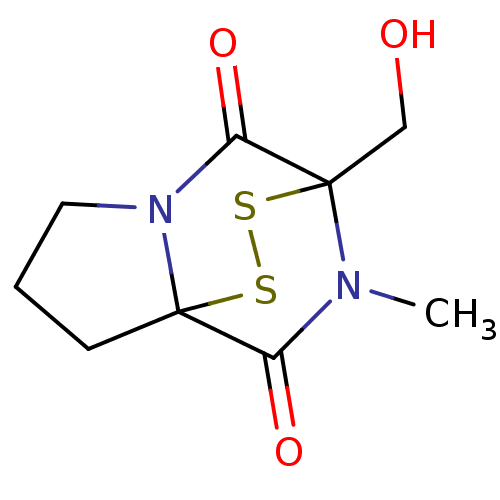 (CHEMBL2315521) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425304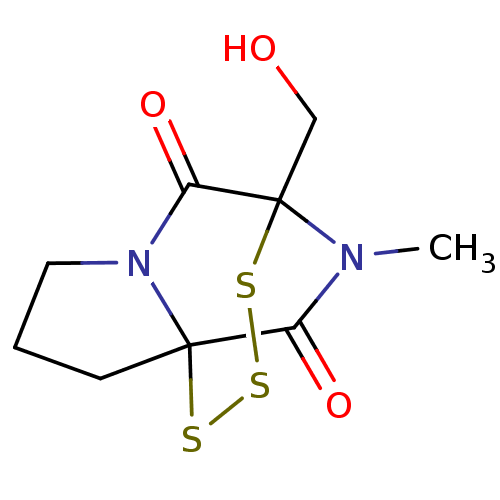 (CHEMBL2315522) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50396029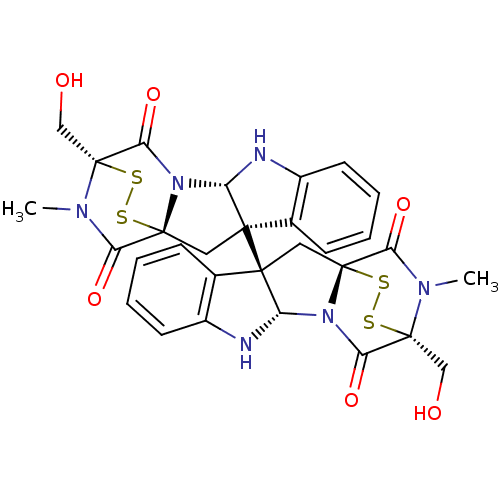 (CHEMBL1222849) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425311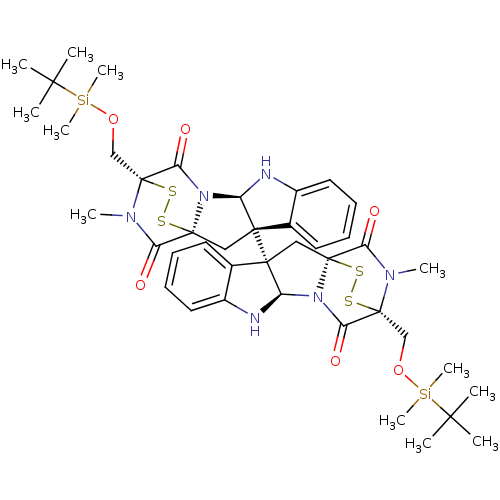 (CHEMBL2315526) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50315537 (CHEMBL1089316 | chaetocin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425306 (CHEMBL2315520) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425307 (CHEMBL2315523) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425310 (CHEMBL2315525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50396028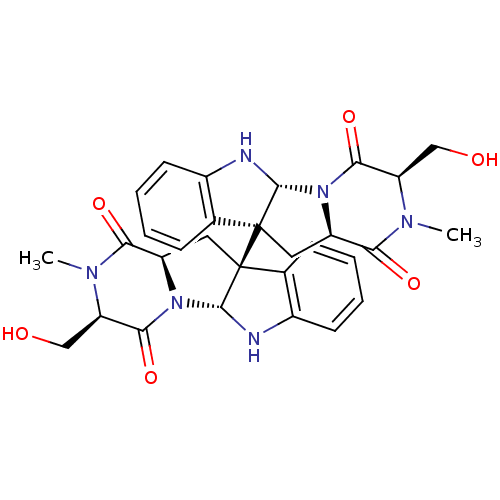 (CHEMBL1222848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425312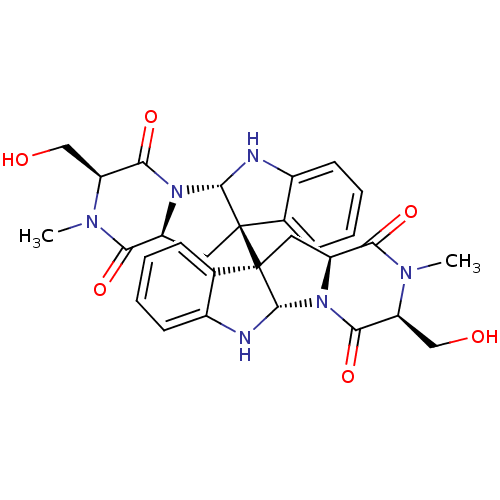 (CHEMBL2315527) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425309 (CHEMBL2315524) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50396028 (CHEMBL1222848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||