

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma1 receptor in Sprague-Dawley rat cerebral membrane after 90 mins by liquid scintillation counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50389433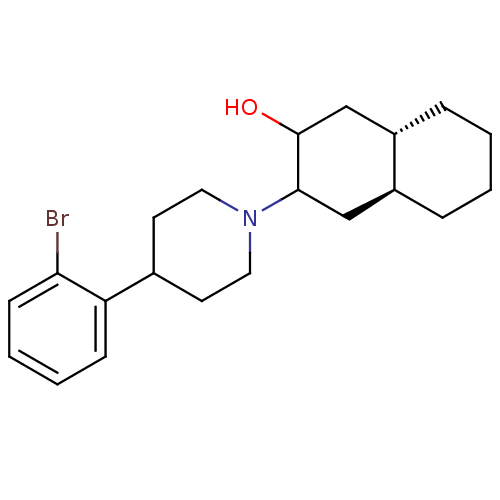 (CHEMBL2064616) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50389435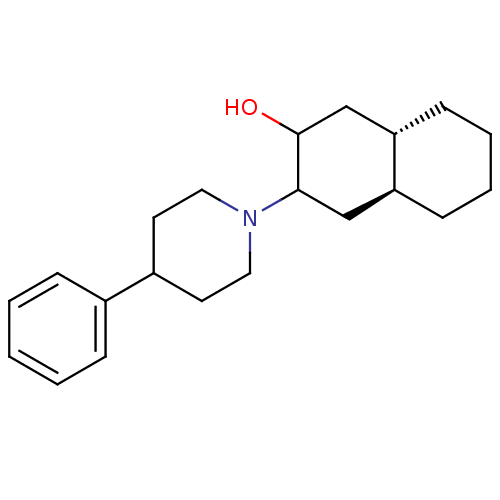 (CHEMBL2064617) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (-)-[3H]vesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 60 mins by liquid scintillation counting in presence of DT... | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50389433 (CHEMBL2064616) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (-)-[3H]vesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 60 mins by liquid scintillation counting in presence of DT... | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50389432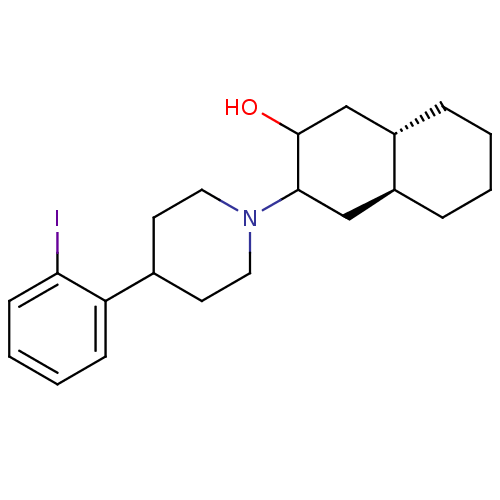 (CHEMBL2064618) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (-)-[3H]vesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 60 mins by liquid scintillation counting in presence of DT... | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50018078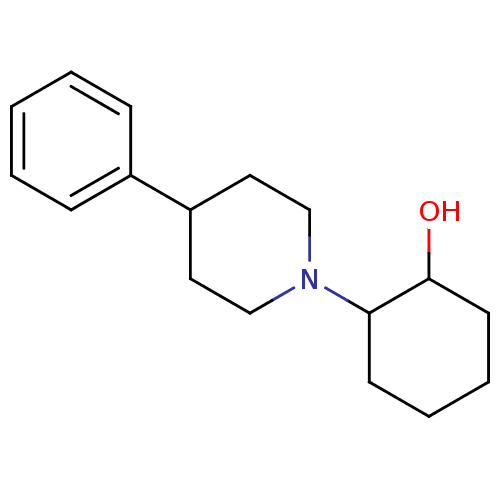 (2-(4-Phenyl-piperidin-1-yl)-cyclohexanol | 2-(4-Ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma1 receptor in Sprague-Dawley rat cerebral membrane after 90 mins by liquid scintillation counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50389432 (CHEMBL2064618) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50018078 (2-(4-Phenyl-piperidin-1-yl)-cyclohexanol | 2-(4-Ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50018078 (2-(4-Phenyl-piperidin-1-yl)-cyclohexanol | 2-(4-Ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (-)-[3H]vesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 60 mins by liquid scintillation counting in presence of DT... | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50389435 (CHEMBL2064617) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma1 receptor in Sprague-Dawley rat cerebral membrane after 90 mins by liquid scintillation counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM81982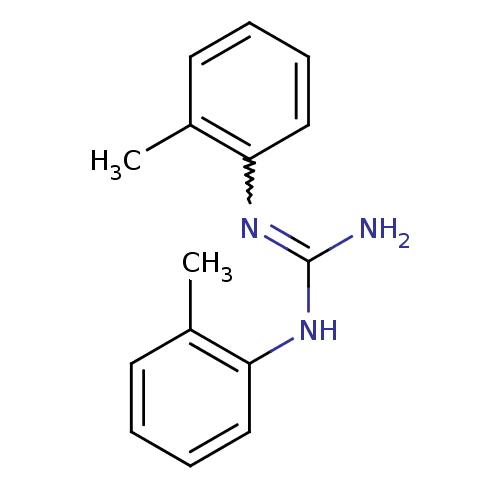 (CAS_97-39-2 | DITOLYLGUANIDINE | DTG | Di-o-tolylg...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma1 receptor in Sprague-Dawley rat cerebral membrane after 90 mins by liquid scintillation counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50389433 (CHEMBL2064616) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma1 receptor in Sprague-Dawley rat cerebral membrane after 90 mins by liquid scintillation counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50389432 (CHEMBL2064618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma1 receptor in Sprague-Dawley rat cerebral membrane after 90 mins by liquid scintillation counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50010096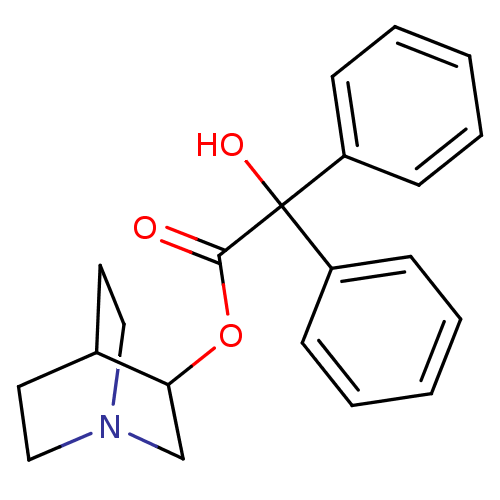 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 582 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM81982 (CAS_97-39-2 | DITOLYLGUANIDINE | DTG | Di-o-tolylg...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50001028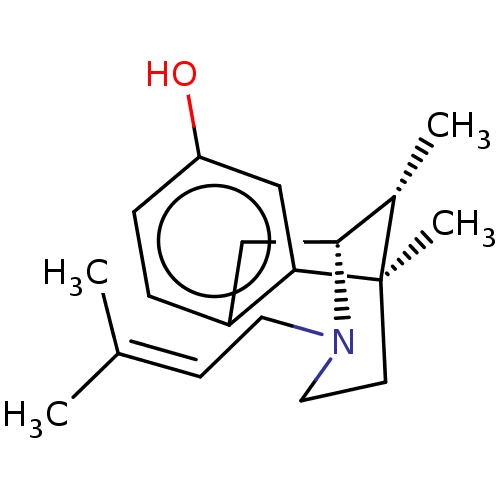 ((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Rattus norvegicus) | BDBM50029051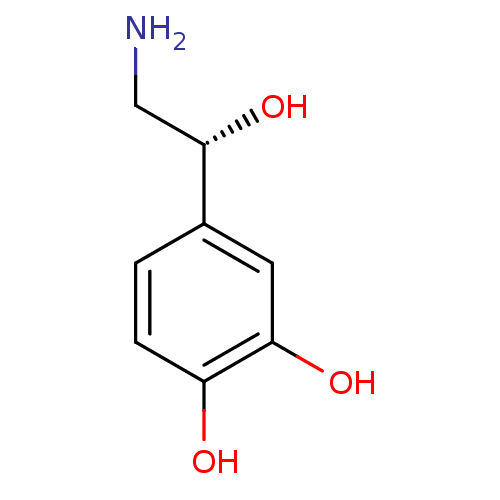 ((-)-arterenol | (-)-noradrenaline | (-)-norepineph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Displacement of [125I]O-iodo-trans-decalinvesamicol from VAChT in Sprague-Dawley rat cerebral membrane after 1 hr by gamma counting | Bioorg Med Chem 20: 4936-41 (2012) Article DOI: 10.1016/j.bmc.2012.06.040 BindingDB Entry DOI: 10.7270/Q24B32DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Rattus norvegicus) | BDBM50322695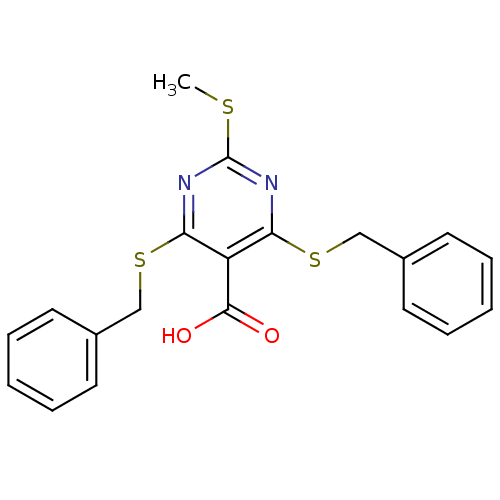 (4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 539 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at rat PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as inhibition of rosiglitazone-induced luciferas... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50322695 (4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at mouse PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as inhibition of rosiglitazone-induced lucifer... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322695 (4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as inhibition of rosiglitazone-induced lucifer... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183267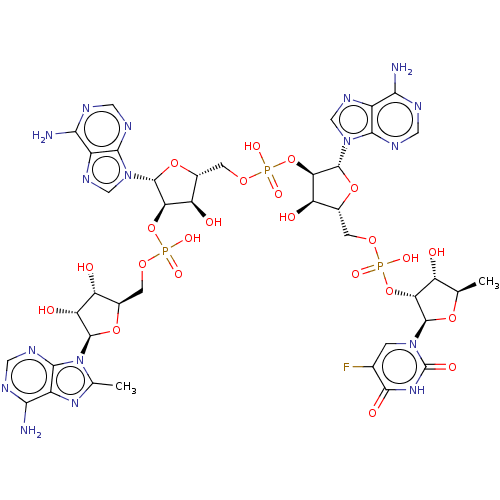 (CHEMBL3817920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183268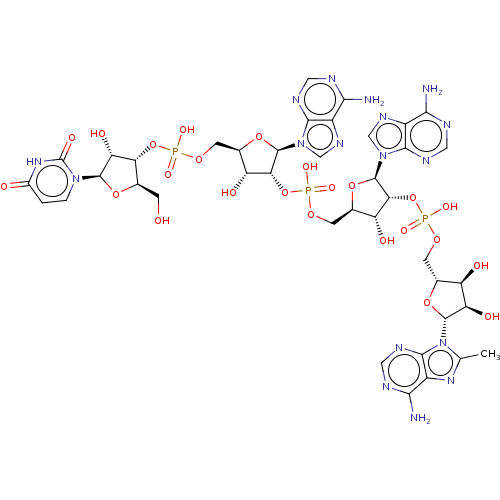 (CHEMBL3818137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 134 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183269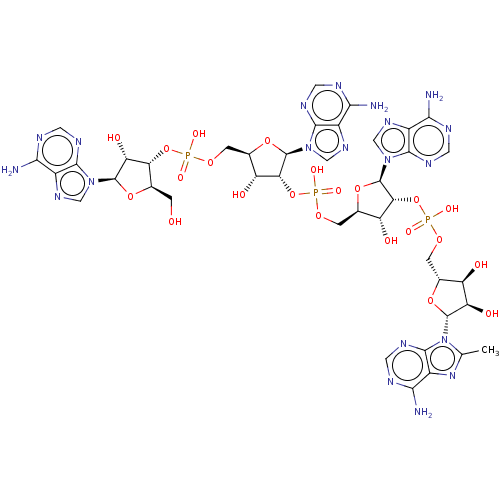 (CHEMBL3818726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183270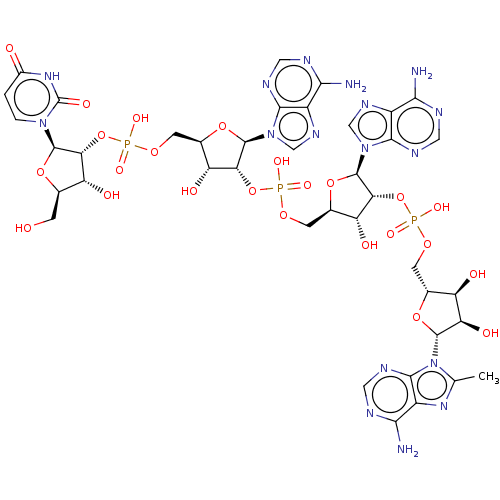 (CHEMBL3818262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183271 (CHEMBL3819183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183278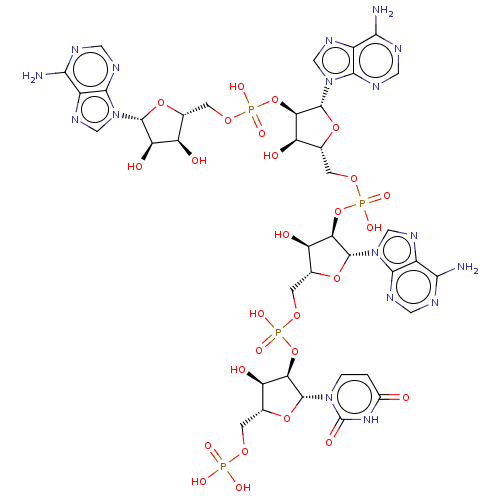 (CHEMBL3819262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50309129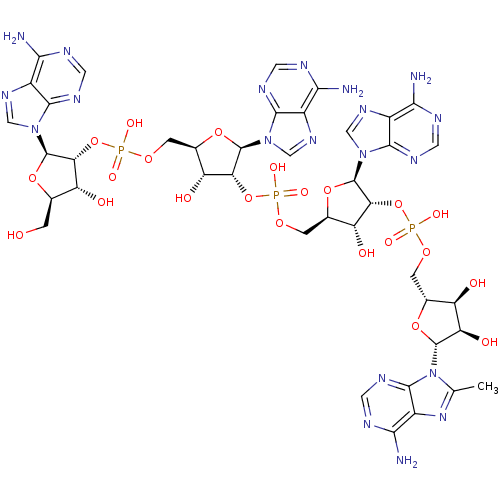 (CHEMBL601938 | {[(2R,3R,4R,5R)-4-[({[(2R,3R,4R,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50183322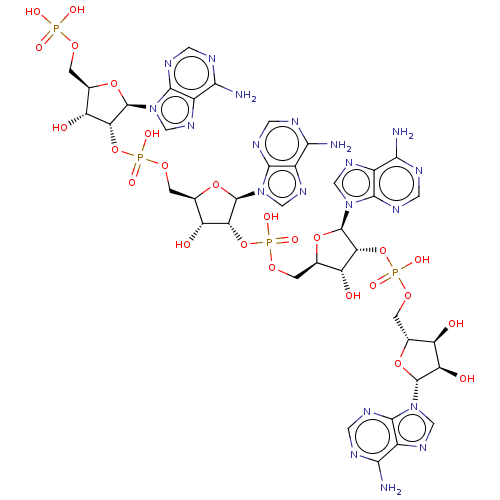 (CHEMBL601940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Induction of recombinant human RNase L activity expressed in Escherichia coli using F-5'-r(C11U2C7)-3' as substrate by polyacrylamide gel electrophor... | Bioorg Med Chem 24: 3870-4 (2016) Article DOI: 10.1016/j.bmc.2016.06.033 BindingDB Entry DOI: 10.7270/Q2RF5WZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50152834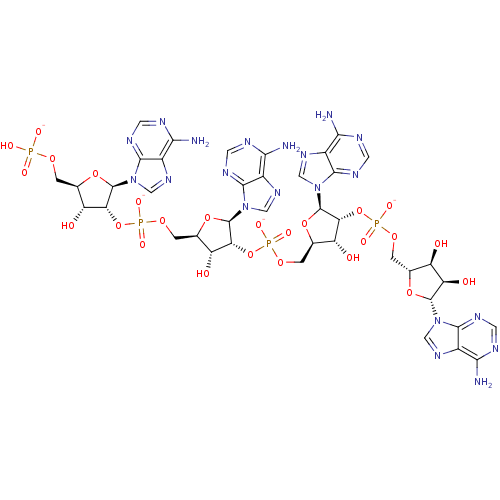 (2''-5--oligoadenylate derivative | [(2R,3R,4R,5R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human RNaseL ANK domain expressed in Escherichia coli assessed as 5' flurescein-r(C11U2C7)-3' RNA cleavage | Bioorg Med Chem Lett 20: 1186-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.005 BindingDB Entry DOI: 10.7270/Q2G73DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50309127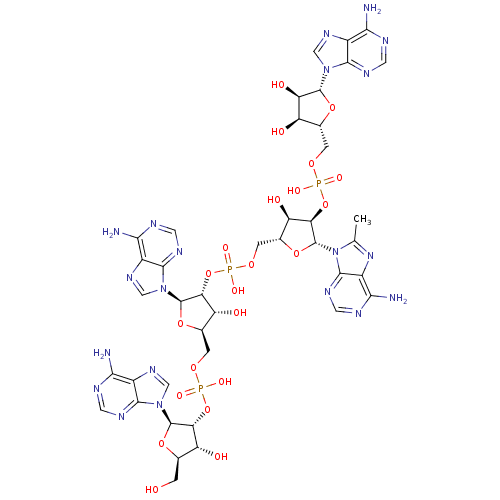 (CHEMBL601939 | {[(2R,3R,4R,5R)-4-[({[(2R,3R,4R,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >50 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human RNaseL ANK domain expressed in Escherichia coli assessed as 5' flurescein-r(C11U2C7)-3' RNA cleavage | Bioorg Med Chem Lett 20: 1186-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.005 BindingDB Entry DOI: 10.7270/Q2G73DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50309128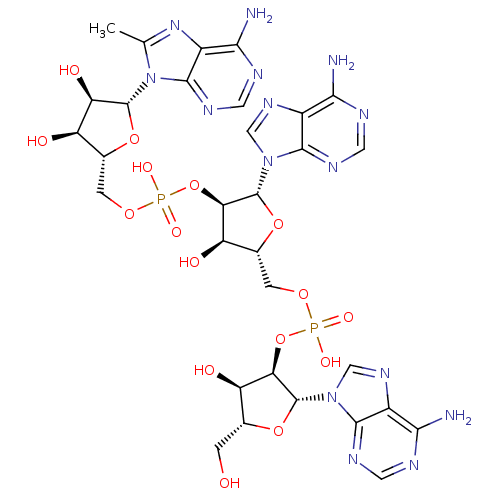 (CHEMBL591445 | {[(2R,3R,4R,5R)-4-[({[(2R,3S,4R,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >50 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human RNaseL ANK domain expressed in Escherichia coli assessed as 5' flurescein-r(C11U2C7)-3' RNA cleavage | Bioorg Med Chem Lett 20: 1186-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.005 BindingDB Entry DOI: 10.7270/Q2G73DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50309129 (CHEMBL601938 | {[(2R,3R,4R,5R)-4-[({[(2R,3R,4R,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human RNaseL ANK domain expressed in Escherichia coli assessed as 5' flurescein-r(C11U2C7)-3' RNA cleavage | Bioorg Med Chem Lett 20: 1186-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.005 BindingDB Entry DOI: 10.7270/Q2G73DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50309130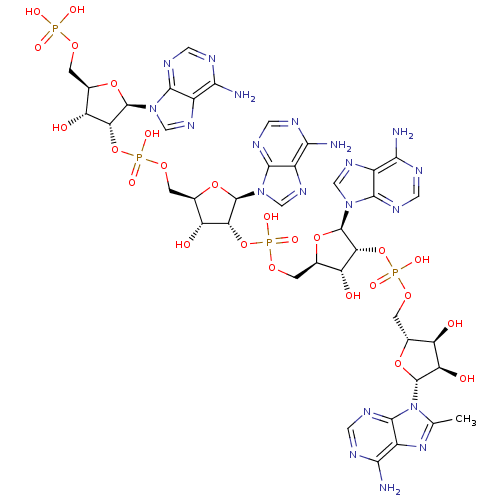 (CHEMBL591446 | {[(2R,3R,4R,5R)-4-[({[(2R,3R,4R,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human RNaseL ANK domain expressed in Escherichia coli assessed as 5' flurescein-r(C11U2C7)-3' RNA cleavage | Bioorg Med Chem Lett 20: 1186-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.005 BindingDB Entry DOI: 10.7270/Q2G73DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322688 (4-(Benzylthio)-2-(methylthio)-6-(4-pyridylmethylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322689 (4-(Benzylthio)-6-(methylamino)-2-(methylthio)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322690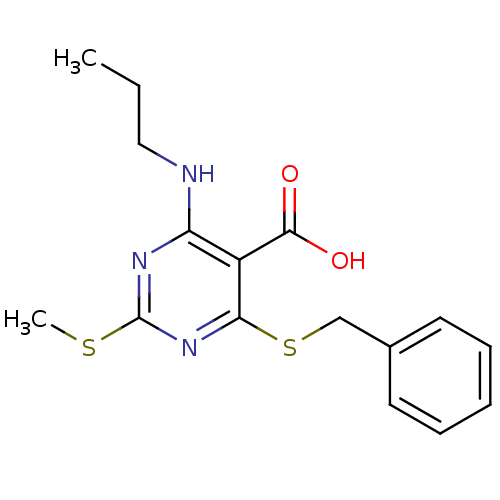 (4-(Benzylthio)-2-(methylthio)-4-(propylamino)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322691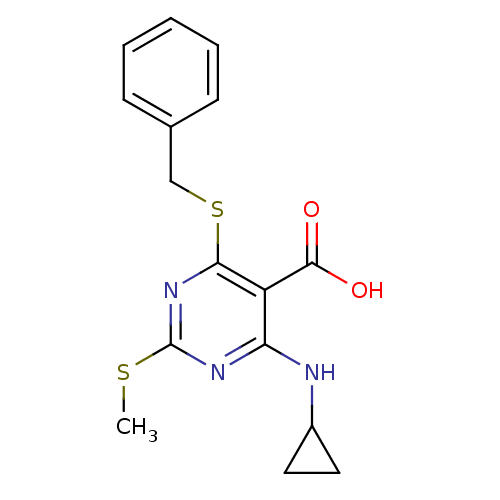 (4-(Benzylthio)-6-(cyclopropylamino)-2-(methylthio)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322692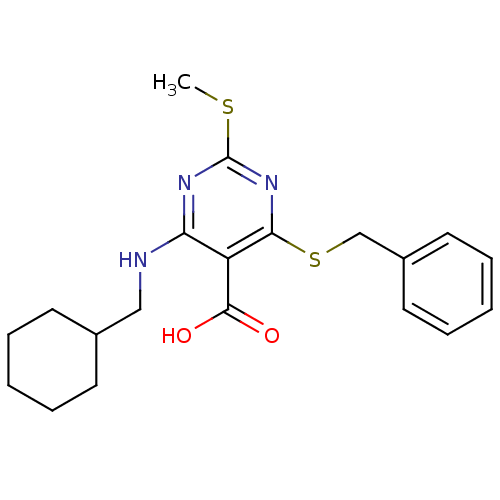 (4-(benzylthio)-6-(cyclohexylmethylamino)-2-(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322693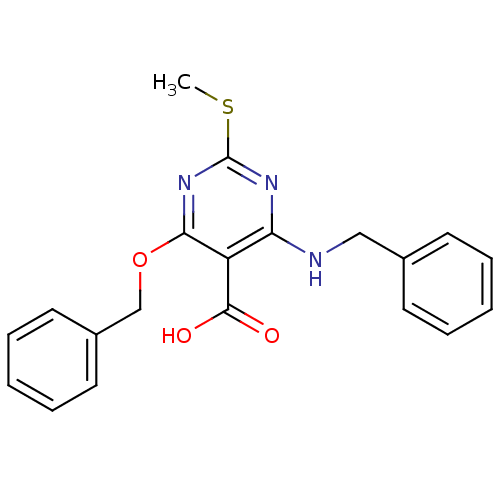 (4-(Benzylamino)-6-(benzyloxy)-2-(methylthio)pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322694 (4-(Benzylamino)-2-(methylthio)-6-(2-thienyl)pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322695 (4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50322695 (4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at mouse PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Rattus norvegicus) | BDBM50322695 (4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at rat PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation a... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322687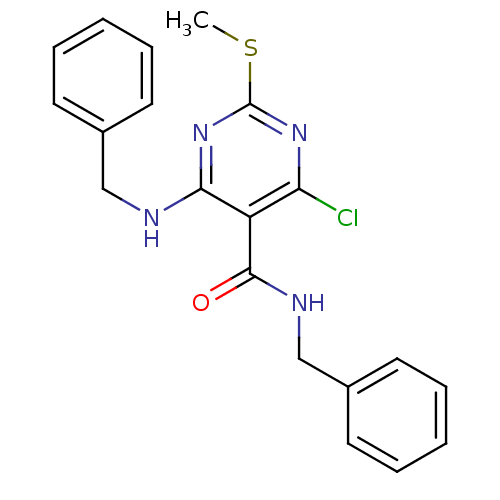 (CHEMBL1172426 | N-benzyl-4-(benzylamino)-6-chloro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322675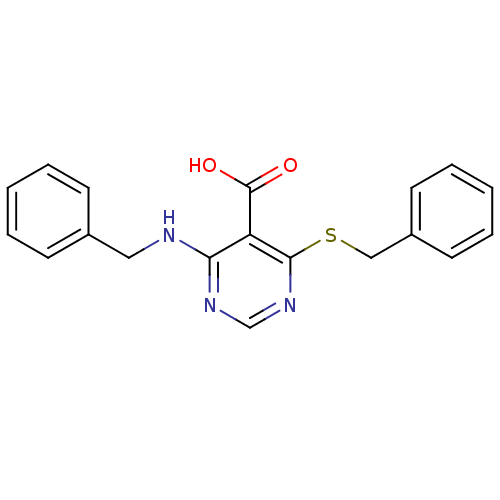 (4-(Benzylamino)-6-(benzylthio)pyrimidine-5-carboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322676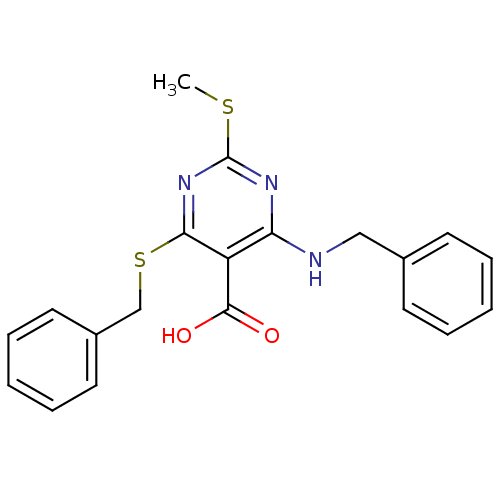 (4-(Benzylamino)-6-(benzylthio)-2-(methylthio)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50322677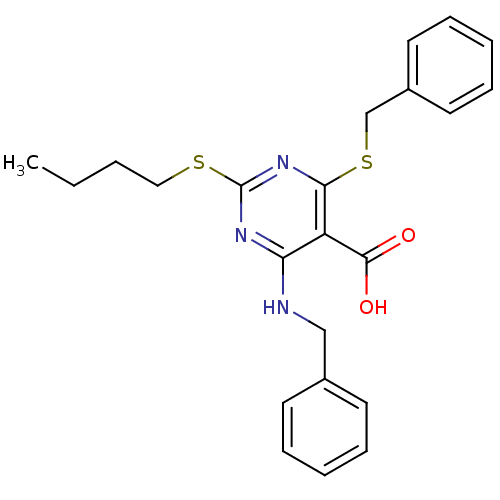 (4-(Benzylamino)-6-(benzylthio)-2-(butylthio)pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as luciferase activity by transactivation... | J Med Chem 53: 5012-24 (2010) Article DOI: 10.1021/jm100443s BindingDB Entry DOI: 10.7270/Q24J0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |