

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163700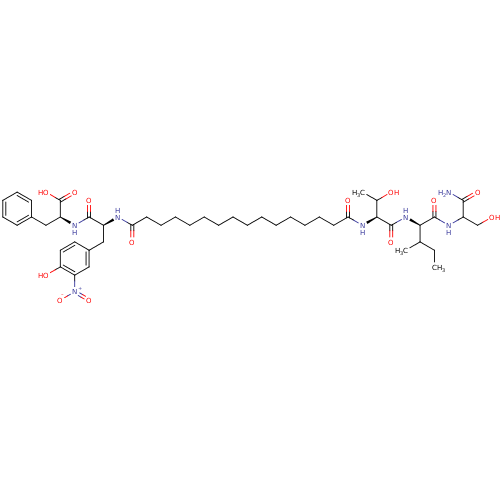 ((S)-2-[(S)-2-(15-{(S)-1-[(R)-1-(1-Carbamoyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163699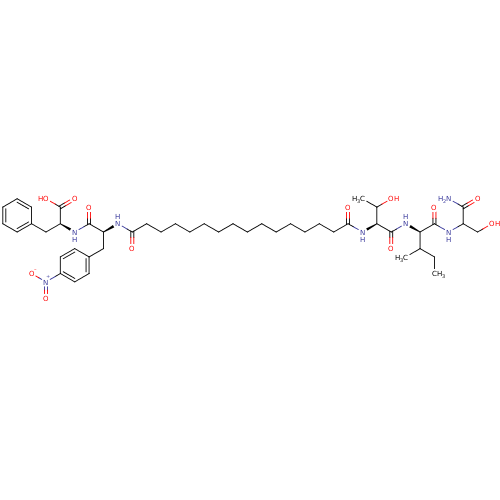 ((S)-2-[(S)-2-(15-{(S)-1-[(R)-1-(1-Carbamoyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163688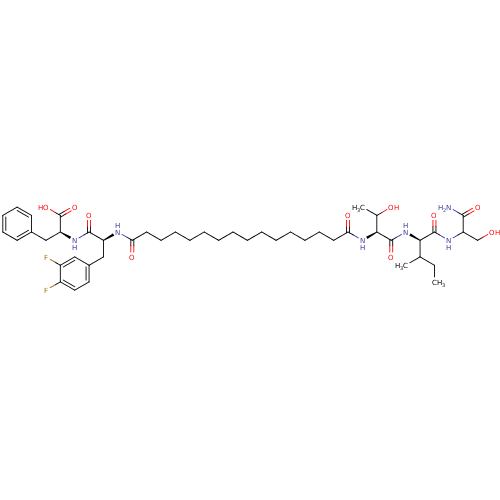 ((S)-2-[(S)-2-(15-{(S)-1-[(R)-1-(1-Carbamoyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163711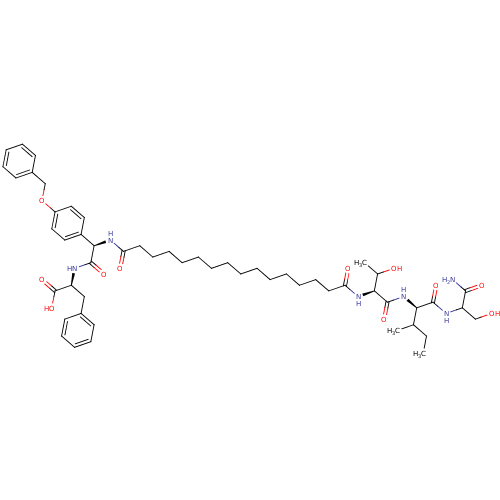 ((S)-2-[(R)-2-(4-Benzyloxy-phenyl)-2-(15-{(S)-1-[(R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370636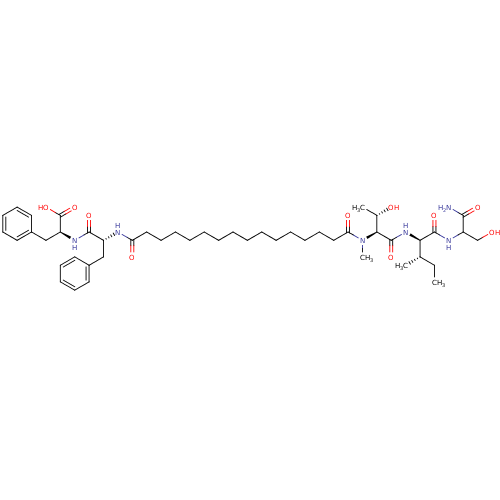 (CHEMBL1791327) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370640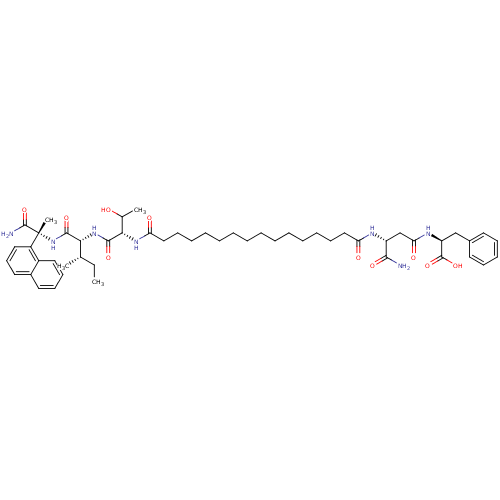 (CHEMBL1791339) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370653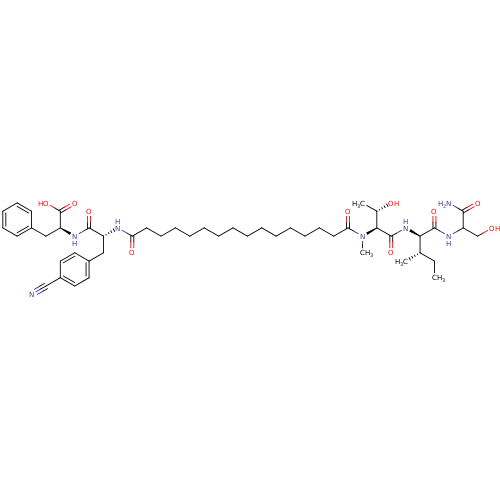 (CHEMBL1791328) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370638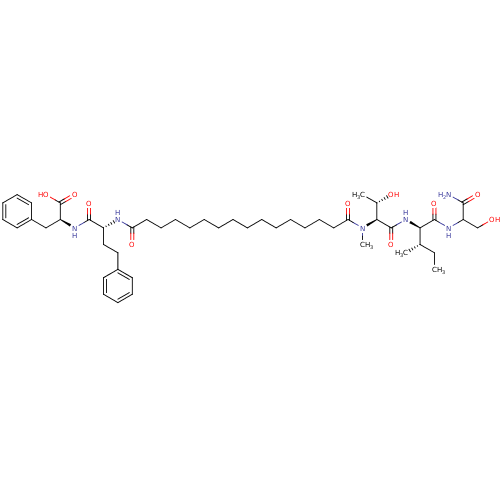 (CHEMBL1791330) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370648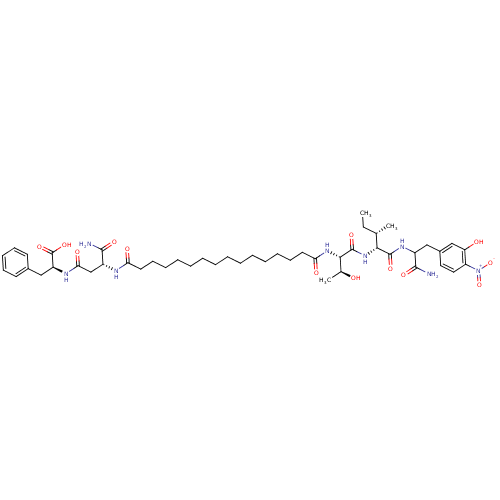 (CHEMBL1791340) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370650 (CHEMBL1791331) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370413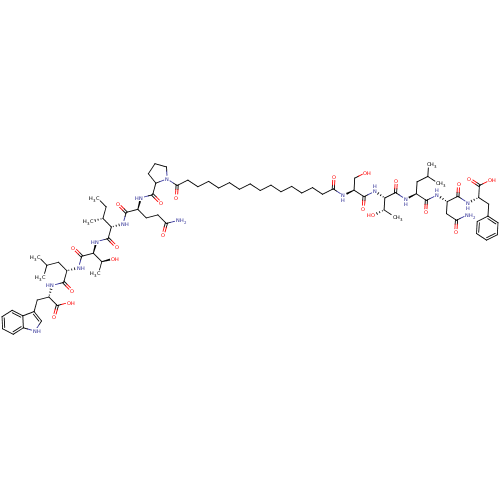 (CHEMBL1791290) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease; (Dimerization inhibitor) | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370413 (CHEMBL1791290) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163694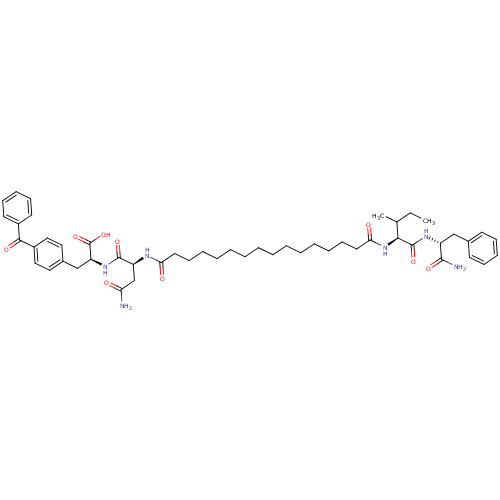 ((S)-3-(4-Benzoyl-phenyl)-2-((S)-3-carbamoyl-2-{15-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163710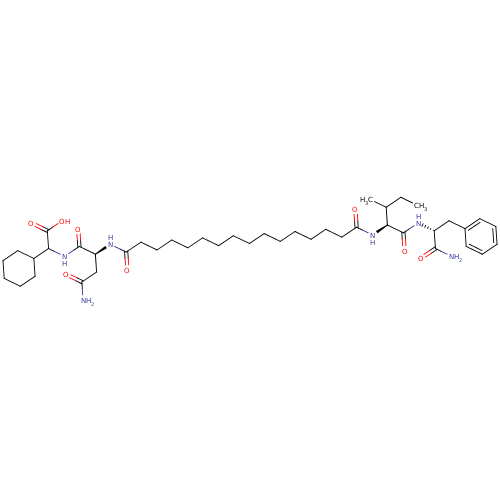 (((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamoyl-2-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163717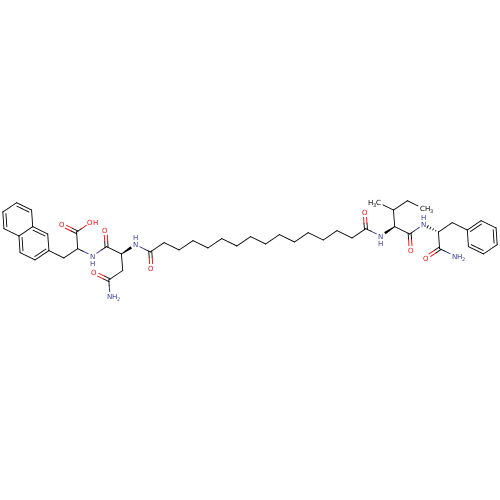 (2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamoyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163696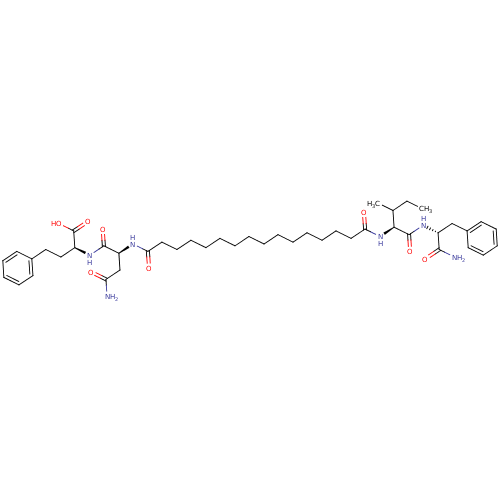 ((S)-2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370643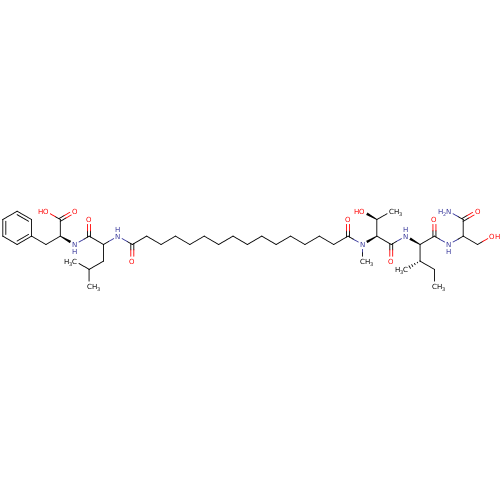 (CHEMBL1791333) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163707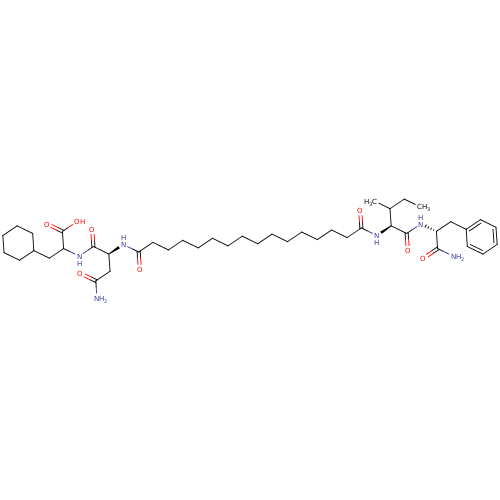 (2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamoyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370654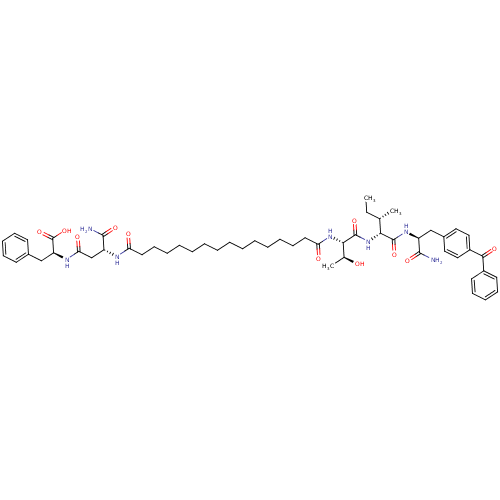 (CHEMBL1791341) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370645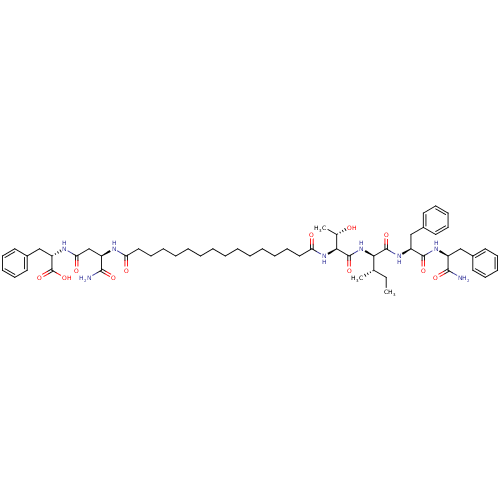 (CHEMBL1791342) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163692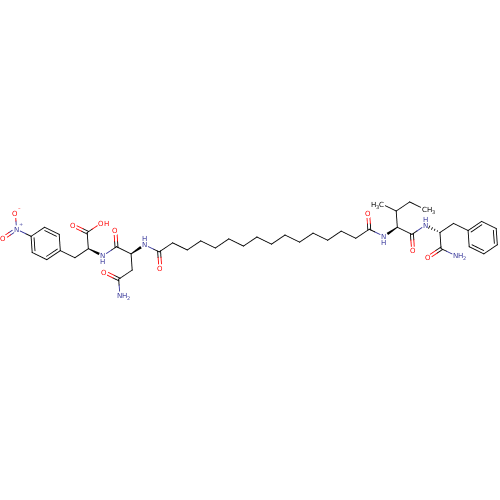 ((S)-2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370641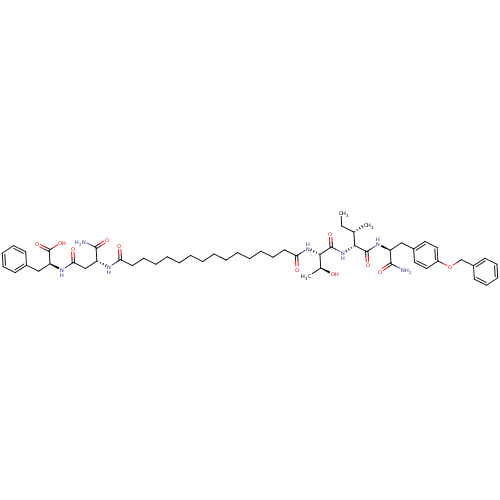 (CHEMBL1791318) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370412 (CHEMBL449777) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163697 ((S)-2-[(R)-3-Carbamoyl-3-(15-{(S)-1-[(S)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150552 ((E)-3-{(S)-1-[((S)-Carbamoyl-{11-[(S)-1-((S)-1-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370639 (CHEMBL1791329) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150540 ((E)-3-{(S)-1-[(S)-1-{11-[(S)-1-((S)-1-Carbamoyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150542 ((E)-3-{(S)-1-[(S)-1-{11-[(S)-1-((S)-1-Carbamoyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163691 ((S)-2-[(R)-3-Carbamoyl-3-(15-{(S)-1-[(R)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150541 ((E)-3-{(S)-1-[(S)-1-(11-{(S)-1-[(S)-1-Carbamoyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370646 (CHEMBL1791332) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163701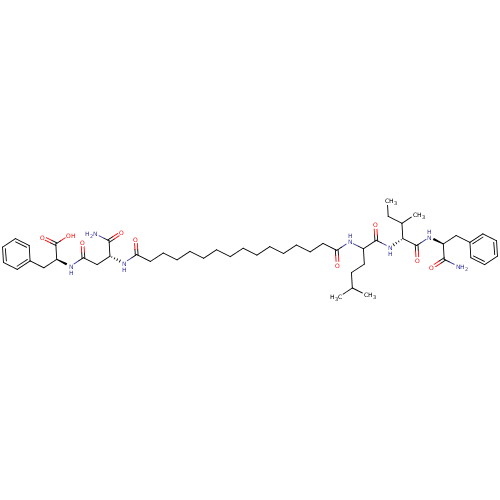 ((S)-2-[(R)-3-Carbamoyl-3-(15-{1-[(R)-1-((S)-1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163691 ((S)-2-[(R)-3-Carbamoyl-3-(15-{(S)-1-[(R)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163719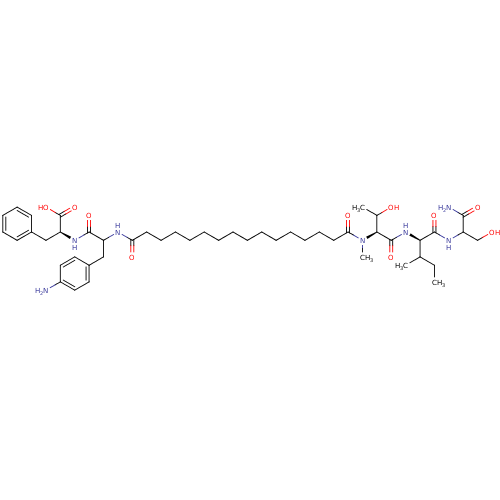 ((S)-2-{3-(4-Amino-phenyl)-2-[15-({(S)-1-[(R)-1-(1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163697 ((S)-2-[(R)-3-Carbamoyl-3-(15-{(S)-1-[(S)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370413 (CHEMBL1791290) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory activity against Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370413 (CHEMBL1791290) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease; (Dimerization inhibitor) | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370633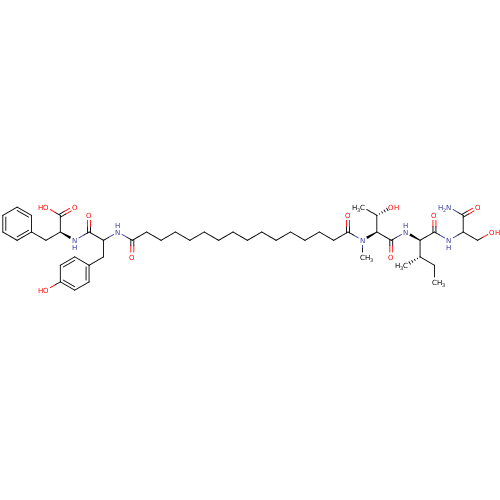 (CHEMBL1791334) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 774 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163726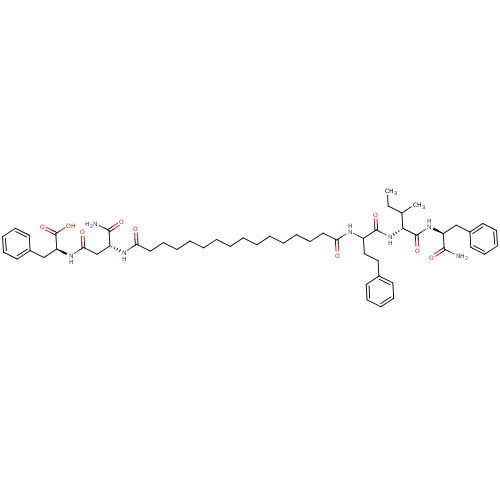 ((S)-2-[(R)-3-Carbamoyl-3-(15-{1-[(R)-1-((S)-1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163727 ((S)-2-((R)-3-Carbamoyl-3-{15-[1-[(R)-1-((S)-1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370637 (CHEMBL1791322) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370647 (CHEMBL1791323) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163716 ((S)-2-[(R)-3-Carbamoyl-3-(15-{1-[(R)-1-((S)-1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370635 (CHEMBL1791324) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163690 ((S)-2-{(R)-3-Carbamoyl-3-[15-({[(R)-1-((S)-1-carba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163713 ((S)-2-[2-[15-({(S)-1-[(R)-1-(1-Carbamoyl-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370649 (CHEMBL1791325) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370642 (CHEMBL1791326) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150543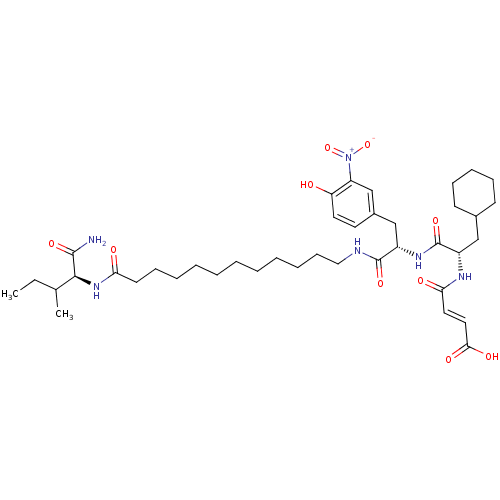 ((E)-3-{(S)-1-[(S)-1-[11-((S)-1-Carbamoyl-2-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory activity against Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370412 (CHEMBL449777) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory activity against Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |