 Found 45 hits with Last Name = 'wang' and Initial = 'yw'
Found 45 hits with Last Name = 'wang' and Initial = 'yw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50590454
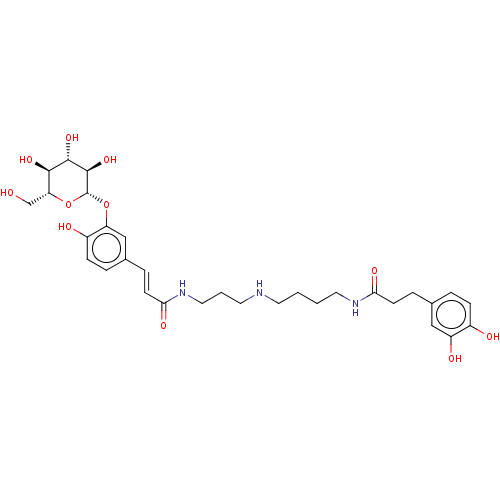
(CHEMBL5195220)Show SMILES OC[C@H]1O[C@@H](Oc2cc(\C=C\C(=O)NCCCNCCCCNC(=O)CCc3ccc(O)c(O)c3)ccc2O)[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50590453
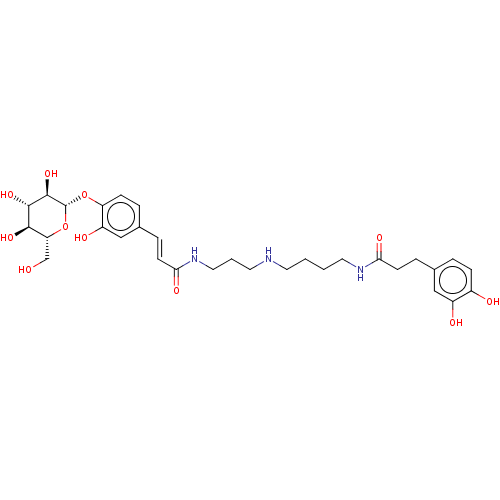
(CHEMBL5186695)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(\C=C\C(=O)NCCCNCCCCNC(=O)CCc3ccc(O)c(O)c3)cc2O)[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50590452
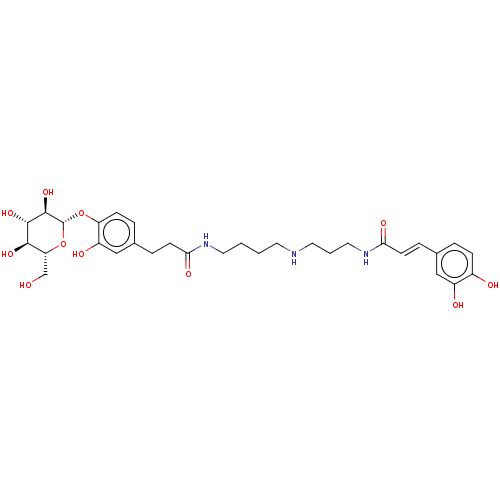
(CHEMBL5206380)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(CCC(=O)NCCCCNCCCNC(=O)\C=C\c3ccc(O)c(O)c3)cc2O)[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600115

(CHEMBL5179721)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3c(F)c(O)c(O)c(O)c3F)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600113
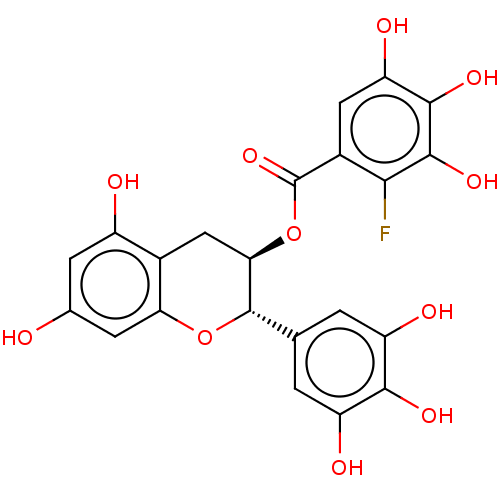
(CHEMBL5173320)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3F)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600122

(CHEMBL5187181)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3F)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600121

(CHEMBL5180939)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614958
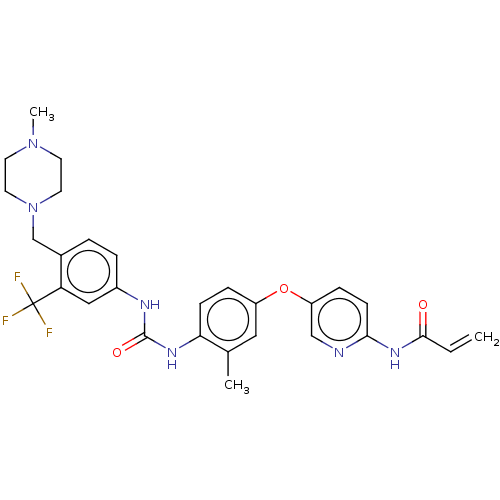
(CHEMBL5283400)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4ccc(NC(=O)C=C)nc4)cc3C)cc2C(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600125

(CHEMBL5183602)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3F)[C@@H](Oc2c1)c1ccc(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614959
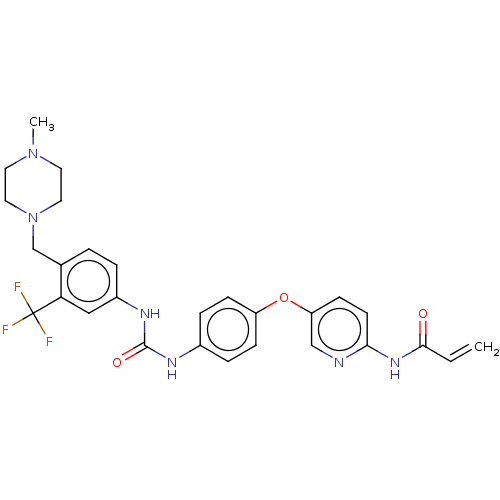
(CHEMBL5275818)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4ccc(NC(=O)C=C)nc4)cc3)cc2C(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600116

(CHEMBL5185250)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)cc3F)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600114

(CHEMBL5196245)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccc(O)c(O)c3O)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600123

(CHEMBL5207075)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3F)[C@@H](Oc2c1)c1cc(O)c(O)cc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50236531
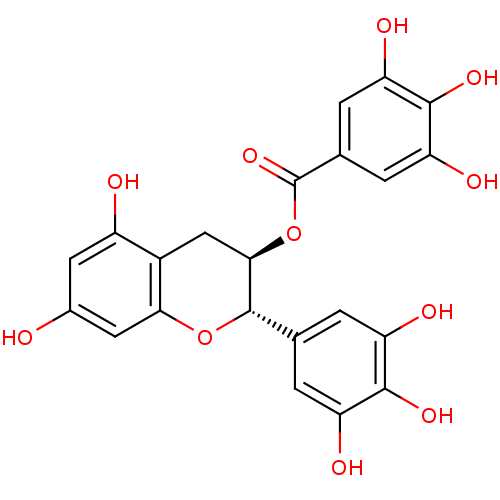
((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600118

(CHEMBL5183972)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(F)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614957
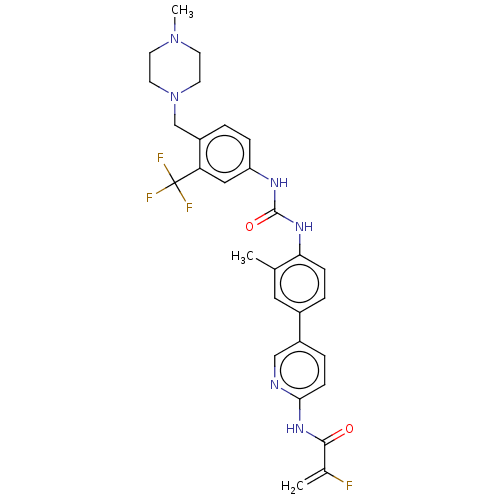
(CHEMBL5279382)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3C)-c3ccc(NC(=O)C(F)=C)nc3)cc2C(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50135168
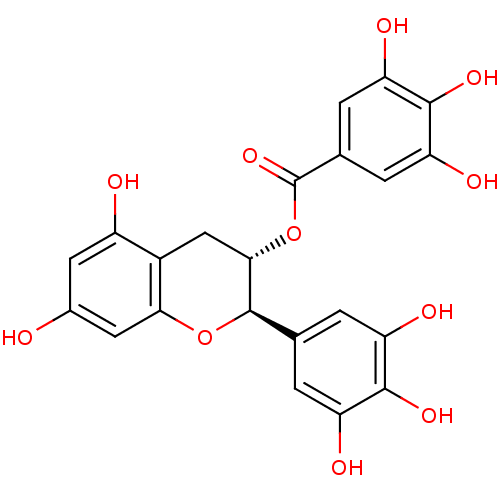
(3,4,5-Trihydroxy-benzoic acid (2R,3S)-5,7-dihydrox...)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600120

(CHEMBL5208564)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)cc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600112
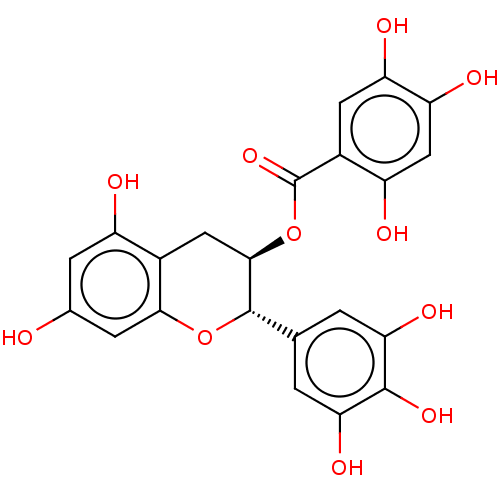
(CHEMBL5173930)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)cc3O)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600127

(CHEMBL5198374)Show SMILES Oc1cc(cc(O)c1O)[C@@H]1Oc2cccc(O)c2C[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614954
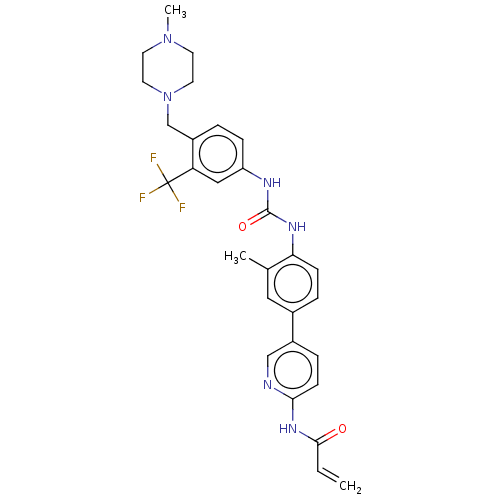
(CHEMBL5279786)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3C)-c3ccc(NC(=O)C=C)nc3)cc2C(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50135169
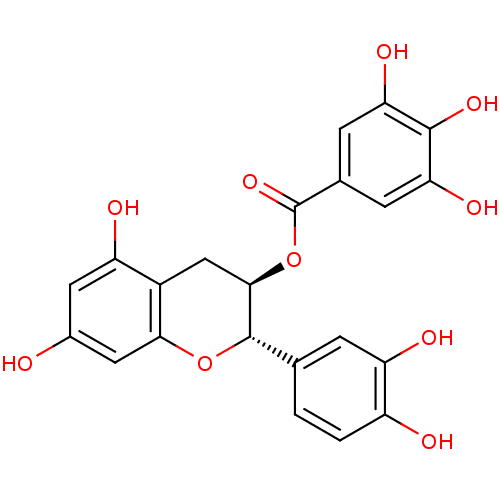
((-)-Catechin gallate | (2S,3R)-2-(3,4-dihydroxyphe...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1ccc(O)c(O)c1 Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614956

(CHEMBL5280112)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3C)-c3ccc(NC(=O)CCl)nc3)cc2C(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600111

(CHEMBL5190113)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3ccc(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614960
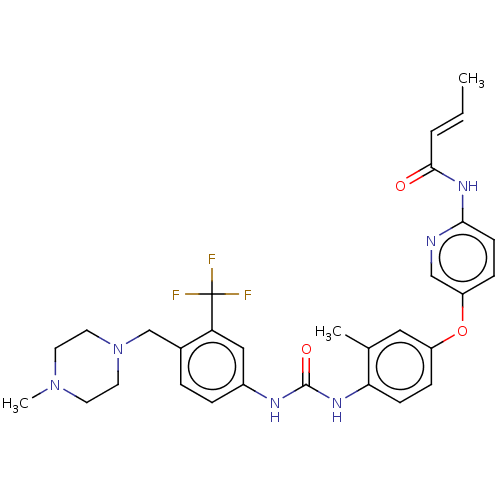
(CHEMBL5278954)Show SMILES C\C=C\C(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3ccc(CN4CCN(C)CC4)c(c3)C(F)(F)F)c(C)c2)cn1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614961
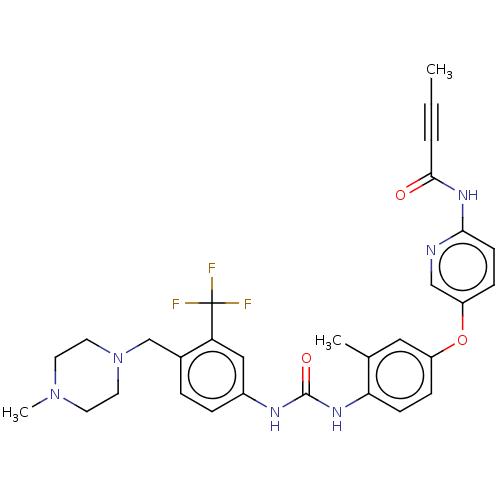
(CHEMBL5266203)Show SMILES CC#CC(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3ccc(CN4CCN(C)CC4)c(c3)C(F)(F)F)c(C)c2)cn1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600124
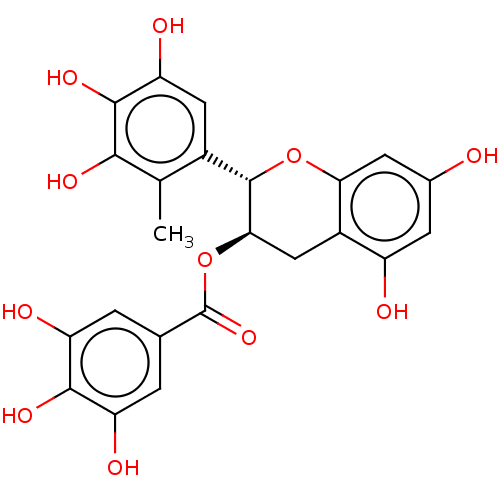
(CHEMBL5180222)Show SMILES Cc1c(O)c(O)c(O)cc1[C@@H]1Oc2cc(O)cc(O)c2C[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50614955
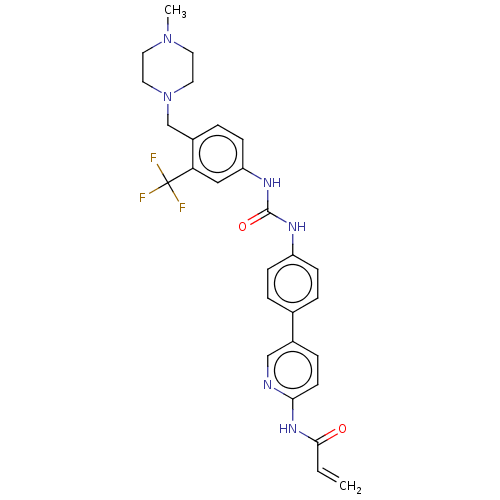
(CHEMBL5282627)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3)-c3ccc(NC(=O)C=C)nc3)cc2C(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600126
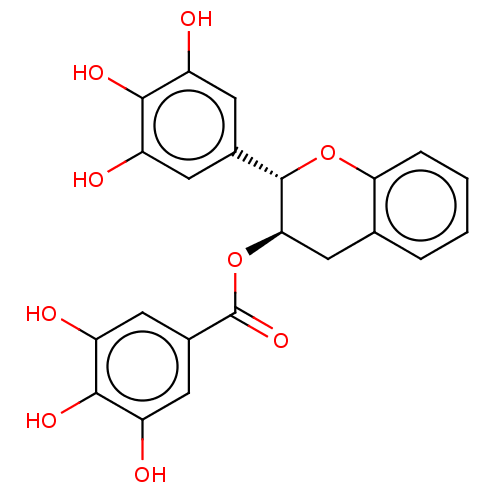
(CHEMBL5205632)Show SMILES Oc1cc(cc(O)c1O)[C@@H]1Oc2ccccc2C[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50296234

((-)-epigallocatechin-3-(3''-O-methyl)gallate | (2R...)Show SMILES COc1cc(cc(O)c1O)C(=O)O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C23H20O11/c1-32-18-5-10(4-16(28)21(18)30)23(31)34-19-8-12-13(25)6-11(24)7-17(12)33-22(19)9-2-14(26)20(29)15(27)3-9/h2-7,19,22,24-30H,8H2,1H3/t19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 429 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600119
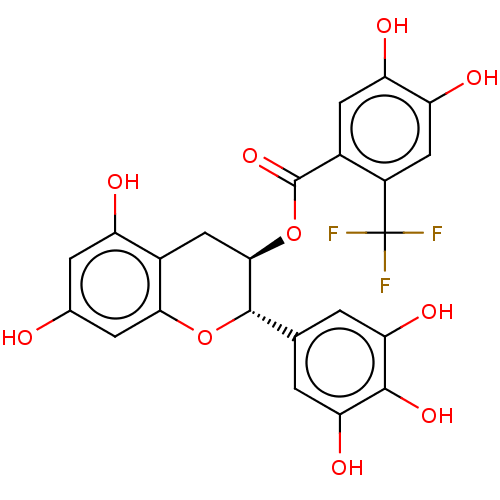
(CHEMBL5191458)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)cc3C(F)(F)F)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50153015

((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 523 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600117

(CHEMBL5173568)Show SMILES Cc1c(O)c(O)ccc1C(=O)O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 553 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50590455
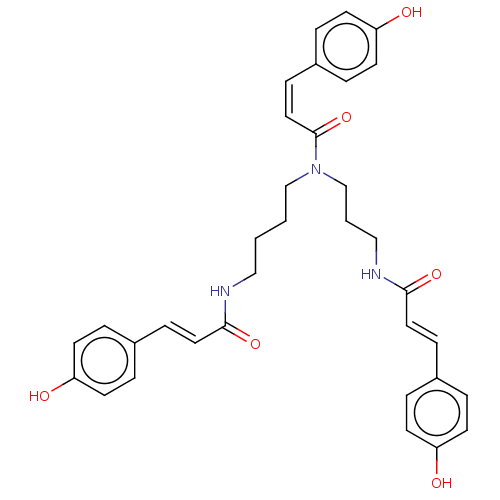
(CHEMBL5175117)Show SMILES Oc1ccc(\C=C\C(=O)NCCCCN(CCCNC(=O)\C=C\c2ccc(O)cc2)C(=O)\C=C/c2ccc(O)cc2)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600128

(CHEMBL5209173)Show SMILES Oc1cc(cc(O)c1O)[C@@H]1Cc2ccccc2C[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50536271

(CHEMBL4556577)Show SMILES CCc1ccccc1NC(=O)CSc1nc(cc(-c2ccc(OC)cc2)c1C#N)-c1ccc(OC)cc1 Show InChI InChI=1S/C30H27N3O3S/c1-4-20-7-5-6-8-27(20)32-29(34)19-37-30-26(18-31)25(21-9-13-23(35-2)14-10-21)17-28(33-30)22-11-15-24(36-3)16-12-22/h5-17H,4,19H2,1-3H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heilongjiang Province Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA assessed as conversion of pyruvate to lactate measuring disappearance of NADH after 10 mins by fluorescence assay |
Bioorg Med Chem Lett 26: 3984-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.083
BindingDB Entry DOI: 10.7270/Q2VD72ZJ |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50536274

(CHEMBL4577861)Show SMILES Cc1ccc(cc1)-c1cc(nc(SCC(=O)Nc2cccc(C)c2)c1C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C28H22FN3OS/c1-18-6-8-20(9-7-18)24-15-26(21-10-12-22(29)13-11-21)32-28(25(24)16-30)34-17-27(33)31-23-5-3-4-19(2)14-23/h3-15H,17H2,1-2H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heilongjiang Province Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA assessed as conversion of pyruvate to lactate measuring disappearance of NADH after 10 mins by fluorescence assay |
Bioorg Med Chem Lett 26: 3984-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.083
BindingDB Entry DOI: 10.7270/Q2VD72ZJ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50600129
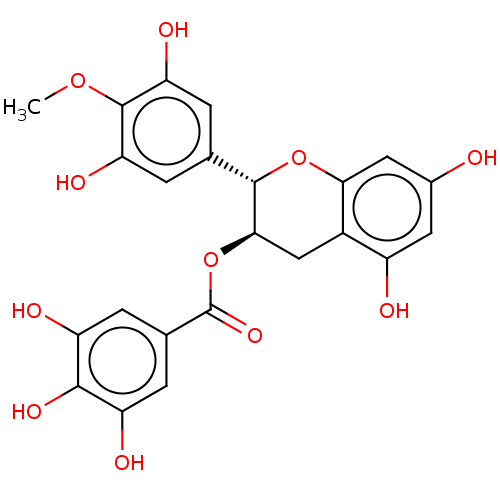
(CHEMBL5195624)Show SMILES COc1c(O)cc(cc1O)[C@@H]1Oc2cc(O)cc(O)c2C[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50536270
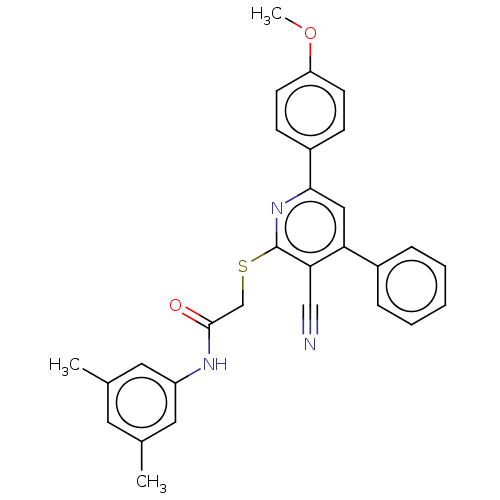
(CHEMBL4537906)Show SMILES COc1ccc(cc1)-c1cc(-c2ccccc2)c(C#N)c(SCC(=O)Nc2cc(C)cc(C)c2)n1 Show InChI InChI=1S/C29H25N3O2S/c1-19-13-20(2)15-23(14-19)31-28(33)18-35-29-26(17-30)25(21-7-5-4-6-8-21)16-27(32-29)22-9-11-24(34-3)12-10-22/h4-16H,18H2,1-3H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heilongjiang Province Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA assessed as conversion of pyruvate to lactate measuring disappearance of NADH after 10 mins by fluorescence assay |
Bioorg Med Chem Lett 26: 3984-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.083
BindingDB Entry DOI: 10.7270/Q2VD72ZJ |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50500505

(CHEMBL3746548)Show SMILES COc1ncc(c(OC)n1)-c1ccc2c(Nc3cccc(c3)C(O)=O)c(cnc2c1)S(N)(=O)=O Show InChI InChI=1S/C22H19N5O6S/c1-32-20-16(10-25-22(27-20)33-2)12-6-7-15-17(9-12)24-11-18(34(23,30)31)19(15)26-14-5-3-4-13(8-14)21(28)29/h3-11H,1-2H3,(H,24,26)(H,28,29)(H2,23,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heilongjiang Province Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA assessed as conversion of pyruvate to lactate measuring disappearance of NADH after 10 mins by fluorescence assay |
Bioorg Med Chem Lett 26: 3984-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.083
BindingDB Entry DOI: 10.7270/Q2VD72ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50536272
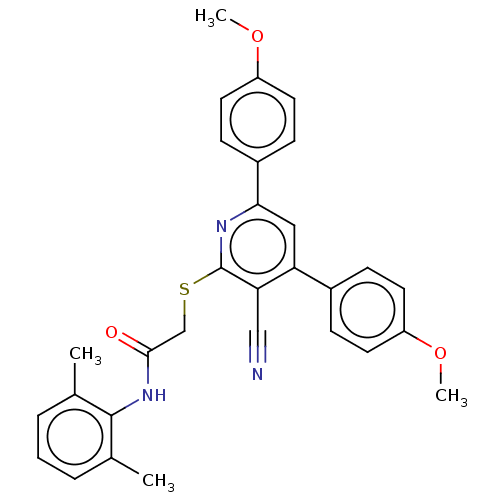
(CHEMBL4592261)Show SMILES COc1ccc(cc1)-c1cc(-c2ccc(OC)cc2)c(C#N)c(SCC(=O)Nc2c(C)cccc2C)n1 Show InChI InChI=1S/C30H27N3O3S/c1-19-6-5-7-20(2)29(19)33-28(34)18-37-30-26(17-31)25(21-8-12-23(35-3)13-9-21)16-27(32-30)22-10-14-24(36-4)15-11-22/h5-16H,18H2,1-4H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heilongjiang Province Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA assessed as conversion of pyruvate to lactate measuring disappearance of NADH after 10 mins by fluorescence assay |
Bioorg Med Chem Lett 26: 3984-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.083
BindingDB Entry DOI: 10.7270/Q2VD72ZJ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50296239

((2S,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)ch...)Show SMILES COc1c(O)cc(cc1O)C(=O)O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C23H20O11/c1-32-22-16(28)4-10(5-17(22)29)23(31)34-19-8-12-13(25)6-11(24)7-18(12)33-21(19)9-2-14(26)20(30)15(27)3-9/h2-7,19,21,24-30H,8H2,1H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50536273

(CHEMBL4556508)Show SMILES COc1ccc(cc1)-c1cc(-c2ccc(OC)cc2)c(C#N)c(SCC(=O)Nc2ccc(C)c(C)c2)n1 Show InChI InChI=1S/C30H27N3O3S/c1-19-5-10-23(15-20(19)2)32-29(34)18-37-30-27(17-31)26(21-6-11-24(35-3)12-7-21)16-28(33-30)22-8-13-25(36-4)14-9-22/h5-16H,18H2,1-4H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heilongjiang Province Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA assessed as conversion of pyruvate to lactate measuring disappearance of NADH after 10 mins by fluorescence assay |
Bioorg Med Chem Lett 26: 3984-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.083
BindingDB Entry DOI: 10.7270/Q2VD72ZJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data