 Found 202 hits with Last Name = 'bai' and Initial = 'z'
Found 202 hits with Last Name = 'bai' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50407503
(CHEMBL5274847)Show InChI InChI=1S/C20H24N4S/c1-2-3-4-5-7-14-10-12-15(13-11-14)25-17-9-6-8-16-18(17)19(21)24-20(22)23-16/h6,8-13H,2-5,7H2,1H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity for carbachol induced contractions in guinea pig ileum against Muscarinic acetylcholine receptor M3 in the presence of mepyrami... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611959
(CHEMBL5276024)Show SMILES [H][C@]1(O[C@@H]2[C@@H](COS(O)(=O)=O)OC([C@@H](OS(O)(=O)=O)[C@H]2OS(O)(=O)=O)C2O[C@H](COS(O)(=O)=O)[C@@H](O[C@@]3([H])O[C@H](COS(O)(=O)=O)[C@@]([H])(O[C@H]4O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@@H]4OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@@H]3OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@@H]2OS(O)(=O)=O)O[C@H](COS(O)(=O)=O)[C@@H](O[C@@]2([H])O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@@H]2OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@@H]1OS(O)(=O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611973

(CHEMBL5282692)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])CC[C@@]([H])(C[C@]4(C)[C@@]3([H])CC[C@]12C)O[C@@H]1O[C@H](COS(O)(=O)=O)[C@@]([H])(O[C@H]2O[C@H](COS(O)(=O)=O)[C@@H](O[C@@]3([H])O[C@H](COS(O)(=O)=O)[C@@]([H])(O[C@H]4O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]4OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]3OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]2OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O)[C@H](C)CCCC(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50093526
(CHEMBL426373 | RK-682)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(O)[C@@H](CO)OC1=O |r,c:17| Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h18,22,24H,2-16H2,1H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431651
(CHEMBL2349236)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(OCc2ccccc2)[C@@H](CO)OC1=O |r,c:17| Show InChI InChI=1S/C28H42O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-24(30)26-27(25(21-29)33-28(26)31)32-22-23-18-15-14-16-19-23/h14-16,18-19,25,29H,2-13,17,20-22H2,1H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Rattus norvegicus) | BDBM50040469
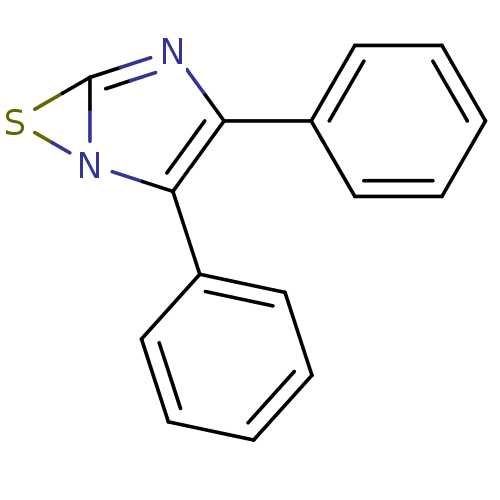
(2,3-Diphenyl-6-thia-1,4-diaza-bicyclo[3.1.0]hexa-2...)Show InChI InChI=1S/C15H10N2S/c1-3-7-11(8-4-1)13-14(17-15(16-13)18-17)12-9-5-2-6-10-12/h1-10H | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the Acyl coenzyme A:cholesterol acyltransferase inhibition using microsomes isolated from the livers of cholesterol fed ra... |
J Med Chem 37: 560-2 (1994)
BindingDB Entry DOI: 10.7270/Q2542MN8 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611974
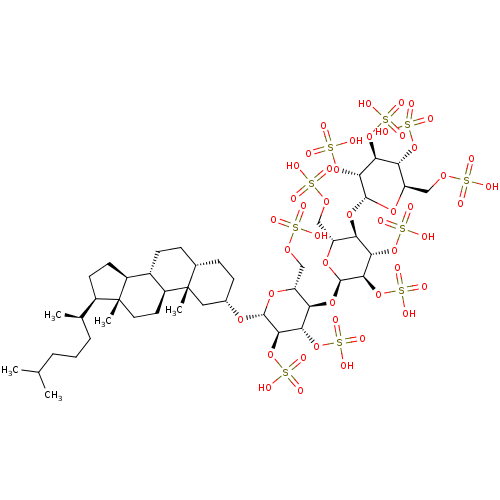
(CHEMBL5271999)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])CC[C@@]([H])(C[C@]4(C)[C@@]3([H])CC[C@]12C)O[C@@H]1O[C@H](COS(O)(=O)=O)[C@@]([H])(O[C@H]2O[C@H](COS(O)(=O)=O)[C@@H](O[C@@]3([H])O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]3OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]2OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O)[C@H](C)CCCC(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611970
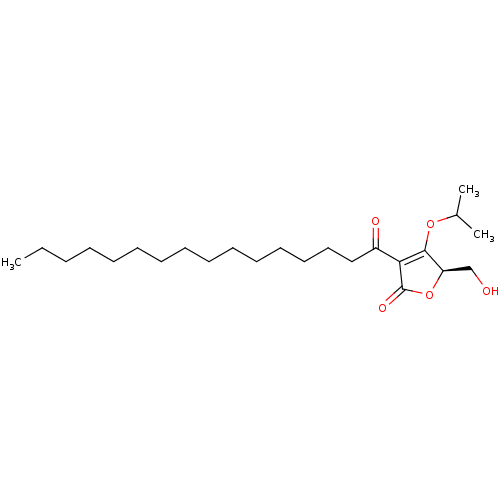
(CHEMBL5286960)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(OC(C)C)[C@@H](CO)OC1=O |r,c:17| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50104680
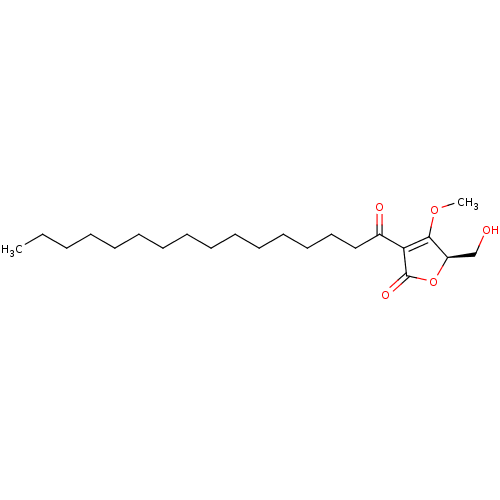
(3-Hexadecanoyl-5-hydroxymethyl-4-methoxy-5H-furan-...)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(OC)[C@@H](CO)OC1=O |c:17| Show InChI InChI=1S/C22H38O5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18(24)20-21(26-2)19(17-23)27-22(20)25/h19,23H,3-17H2,1-2H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147546
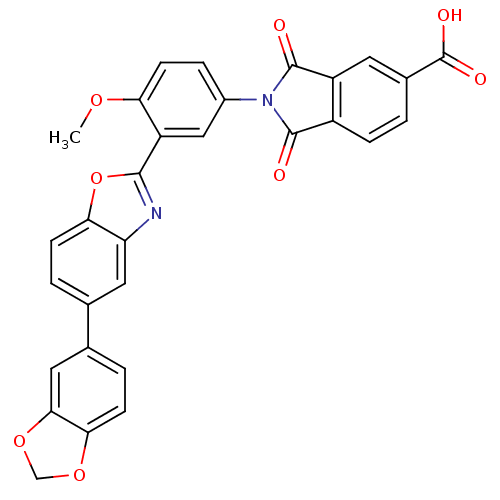
(2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H18N2O8/c1-37-23-9-5-18(32-28(33)19-6-2-17(30(35)36)10-20(19)29(32)34)13-21(23)27-31-22-11-15(3-7-24(22)40-27)16-4-8-25-26(12-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611966
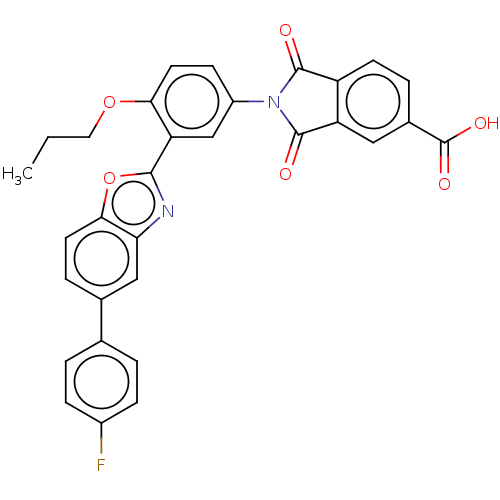
(CHEMBL5277782)Show SMILES CCCOc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(F)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611968

(CHEMBL5266599)Show SMILES COc1ccc(cc1Br)C(=O)Nc1ccc(Nc2ccc(cc2)-c2nc3ccccc3[nH]2)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611965
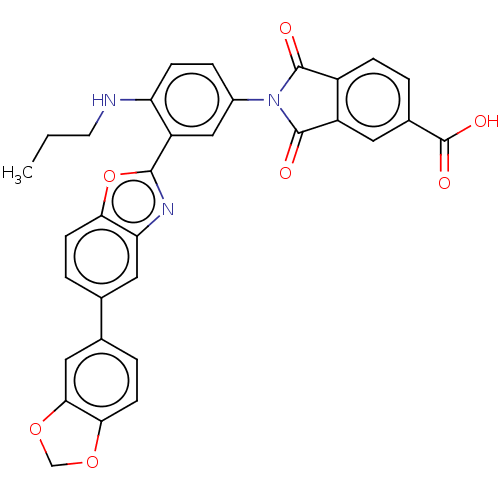
(CHEMBL5271688)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611969
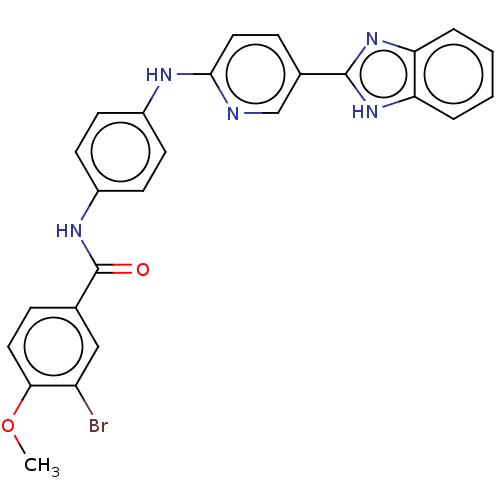
(CHEMBL5265781)Show SMILES COc1ccc(cc1Br)C(=O)Nc1ccc(Nc2ccc(cn2)-c2nc3ccccc3[nH]2)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611961
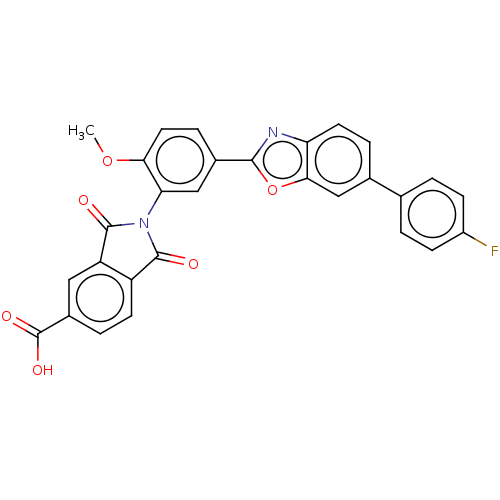
(CHEMBL5280877)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2ccc(cc2o1)-c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611962
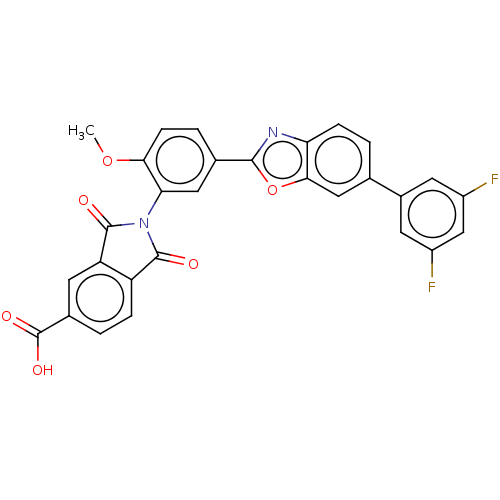
(CHEMBL5269073)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2ccc(cc2o1)-c1cc(F)cc(F)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611963
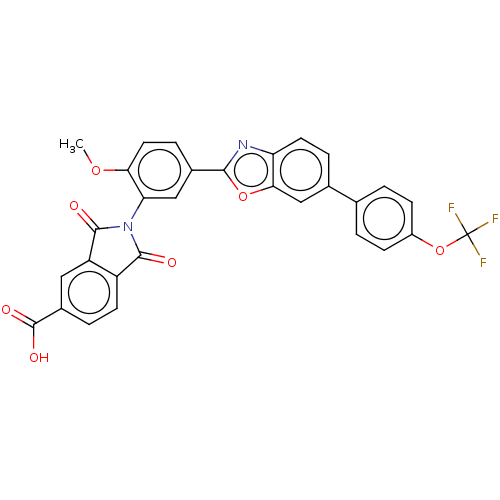
(CHEMBL5275647)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2ccc(cc2o1)-c1ccc(OC(F)(F)F)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611975
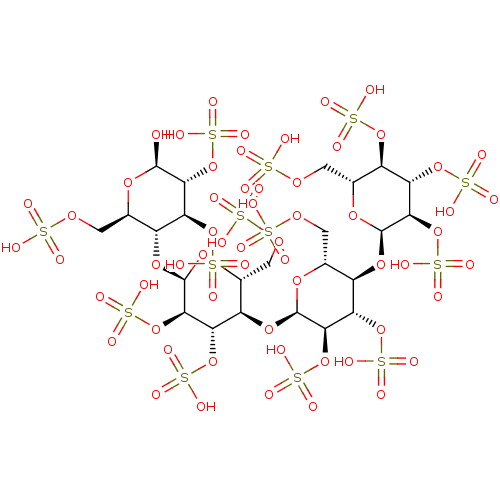
(CHEMBL5276166)Show SMILES [H][C@]1(O[C@@H]2[C@@H](COS(O)(=O)=O)O[C@H](O[C@]3([H])[C@@H](COS(O)(=O)=O)O[C@@H](O)[C@H](OS(O)(=O)=O)[C@H]3OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]2OS(O)(=O)=O)O[C@H](COS(O)(=O)=O)[C@@]([H])(O[C@H]2O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]2OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611967
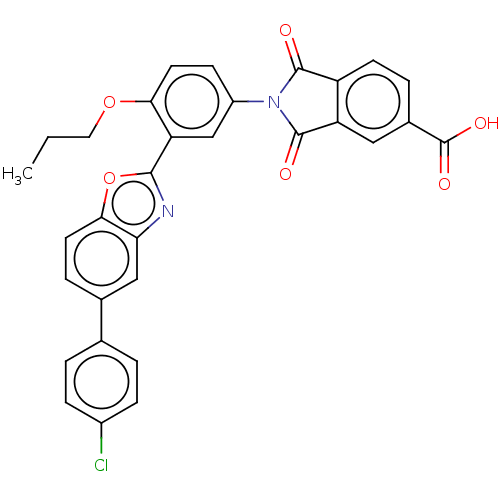
(CHEMBL5281679)Show SMILES CCCOc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain tubulin polymerization |
Bioorg Med Chem Lett 25: 631-4 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.004
BindingDB Entry DOI: 10.7270/Q2DN46PV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heparanase
(Homo sapiens (Human)) | BDBM50378647
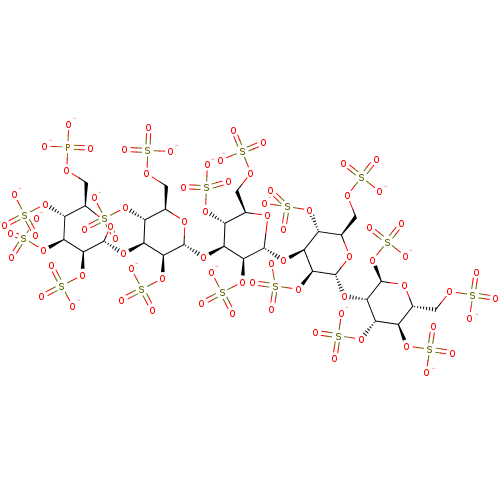
(CHEMBL1627122 | PI-88)Show SMILES [O-]P([O-])(=O)OC[C@H]1O[C@H](O[C@H]2[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]3[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]4[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@@H]5[C@@H](OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C30H53O77PS16/c31-108(32,33)82-1-6-14(99-116(55,56)57)20(102-119(64,65)66)25(106-123(76,77)78)29(87-6)94-17-12(97-114(49,50)51)8(3-84-110(37,38)39)89-27(23(17)104-121(70,71)72)92-16-11(96-113(46,47)48)7(2-83-109(34,35)36)88-26(22(16)103-120(67,68)69)93-18-13(98-115(52,53)54)9(4-85-111(40,41)42)90-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)15(100-117(58,59)60)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H2,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-18/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50407502
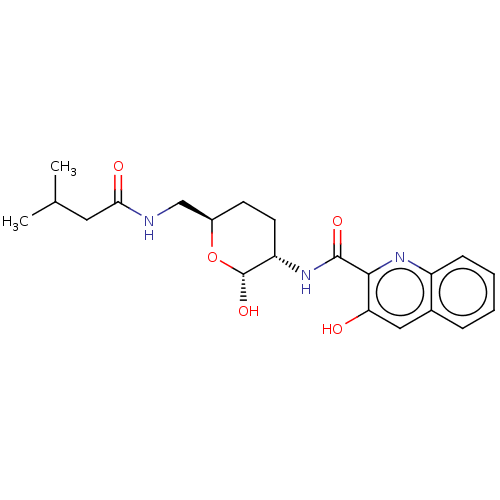
(CHEMBL5285484)Show InChI InChI=1S/C14H13N5S/c15-8-4-6-9(7-5-8)20-11-3-1-2-10-12(11)13(16)19-14(17)18-10/h1-7H,15H2,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity for carbachol induced contractions in guinea pig ileum against Muscarinic acetylcholine receptor M3 in the presence of mepyrami... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50407501
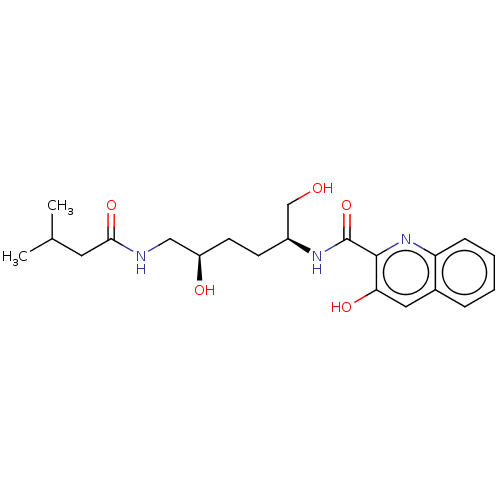
(CHEMBL5266540)Show InChI InChI=1S/C15H14N4OS/c1-20-9-4-2-5-10(8-9)21-12-7-3-6-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tetsed for Antagonistic activity at histamine H3 receptor in guinea pig ileal longitudinal muscle in the presence of mepyramine at a concentration of... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50042033
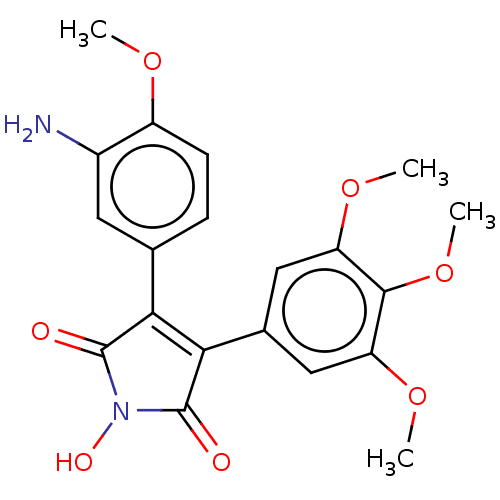
(CHEMBL3360145)Show SMILES COc1ccc(cc1N)C1=C(C(=O)N(O)C1=O)c1cc(OC)c(OC)c(OC)c1 |t:10| Show InChI InChI=1S/C20H20N2O7/c1-26-13-6-5-10(7-12(13)21)16-17(20(24)22(25)19(16)23)11-8-14(27-2)18(29-4)15(9-11)28-3/h5-9,25H,21H2,1-4H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain tubulin polymerization |
Bioorg Med Chem Lett 25: 631-4 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.004
BindingDB Entry DOI: 10.7270/Q2DN46PV |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50405558
(CHEMBL3347523)Show InChI InChI=1S/C19H19N2/c20-12-15-21-13-10-16(11-14-21)8-9-18-6-3-5-17-4-1-2-7-19(17)18/h1-11,13-14H,12,15,20H2/q+1/b9-8+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611980
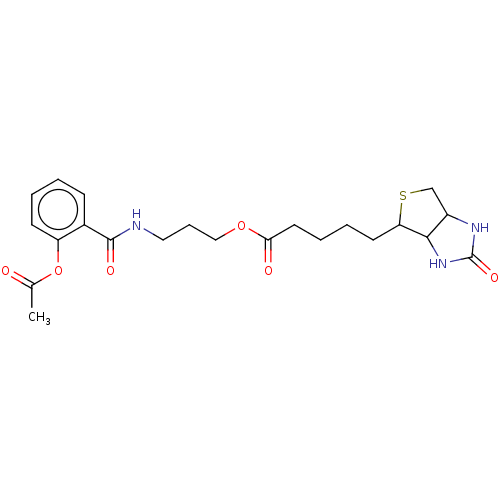
(CHEMBL5288048)Show SMILES CC(=O)Oc1ccccc1C(=O)NCCCOC(=O)CCCCC1SCC2NC(=O)NC12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611972
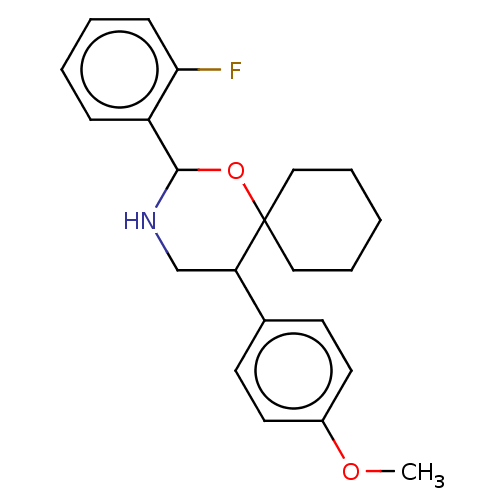
(CHEMBL5266899) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611976
(CHEMBL5269056)Show SMILES [H][C@]1(O[C@@H]2[C@@H](COS(O)(=O)=O)O[C@H](O[C@]3([H])[C@@H](COS(O)(=O)=O)O[C@@H](O)[C@H](OS(O)(=O)=O)[C@H]3OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]2OS(O)(=O)=O)O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50407502

(CHEMBL5285484)Show InChI InChI=1S/C14H13N5S/c15-8-4-6-9(7-5-8)20-11-3-1-2-10-12(11)13(16)19-14(17)18-10/h1-7H,15H2,(H4,16,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Maximum response (E max) against Histamine H1 receptor in rat aorta |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Rattus norvegicus) | BDBM50040468
(2,6-Bis(1-methylethyl)phenyl[[2,6-Bis(1-methylethy...)Show SMILES CC(C)c1cccc(C(C)C)c1OC(=O)[N-]S(=O)(=O)Oc1c(cccc1C(C)C)C(C)C Show InChI InChI=1S/C25H35NO5S/c1-15(2)19-11-9-12-20(16(3)4)23(19)30-25(27)26-32(28,29)31-24-21(17(5)6)13-10-14-22(24)18(7)8/h9-18H,1-8H3,(H,26,27)/p-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the Acyl coenzyme A:cholesterol acyltransferase inhibition using microsomes isolated from the livers of cholesterol fed ra... |
J Med Chem 37: 560-2 (1994)
BindingDB Entry DOI: 10.7270/Q2542MN8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50407501

(CHEMBL5266540)Show InChI InChI=1S/C15H14N4OS/c1-20-9-4-2-5-10(8-9)21-12-7-3-6-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tetsed for Antagonistic activity at histamine H3 receptor in guinea pig ileal longitudinal muscle in the presence of mepyramine at a concentration of... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147526
(2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(c1)-c1nc2ccccc2o1 Show InChI InChI=1S/C22H12N2O5/c25-20-15-9-8-13(22(27)28)11-16(15)21(26)24(20)14-5-3-4-12(10-14)19-23-17-6-1-2-7-18(17)29-19/h1-11H,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147543
(2-(5-Benzooxazol-2-yl-2-methoxy-phenyl)-1,3-dioxo-...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2ccccc2o1 Show InChI InChI=1S/C23H14N2O6/c1-30-19-9-7-12(20-24-16-4-2-3-5-18(16)31-20)11-17(19)25-21(26)14-8-6-13(23(28)29)10-15(14)22(25)27/h2-11H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611964
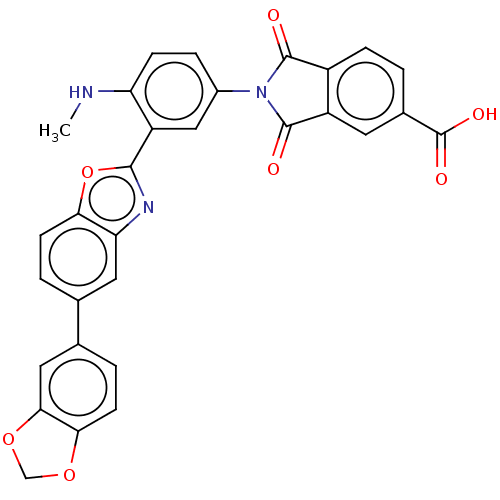
(CHEMBL5288229)Show SMILES CNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611960
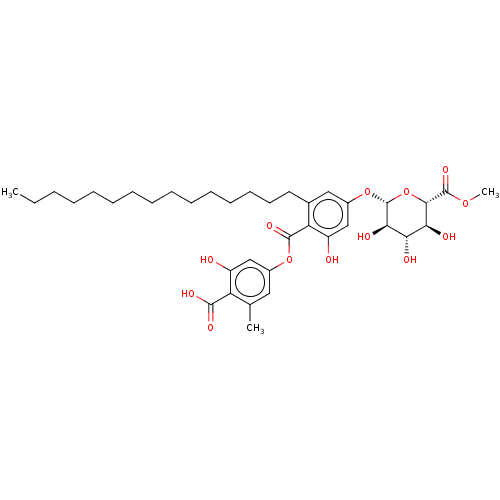
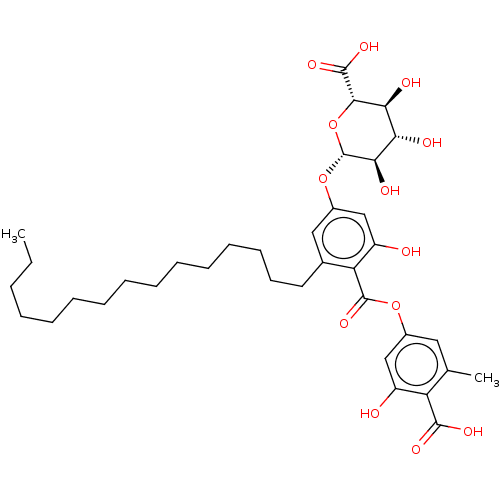
(CHEMBL5275830)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(=O)OC)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50597430
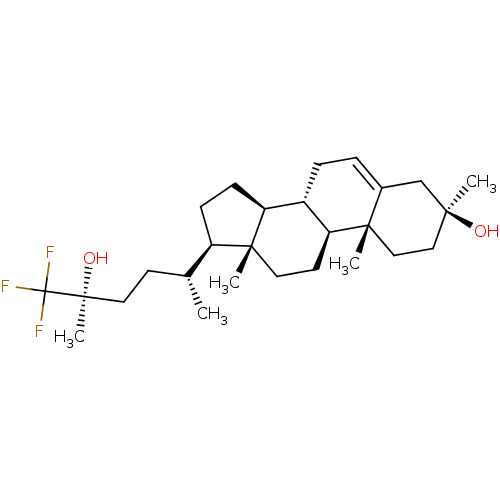
(DALZANEMDOR | Dalzanemdor)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@](C)(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@](C)(O)C(F)(F)F |r,t:9| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00313
BindingDB Entry DOI: 10.7270/Q2XK8KM0 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611958
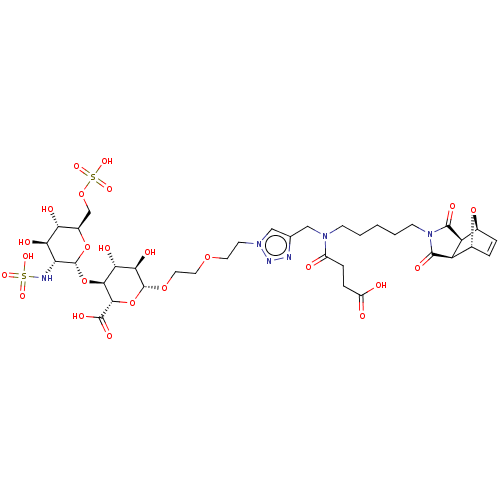
(CHEMBL5277725)Show SMILES [H][C@]12O[C@]([H])(C=C1)[C@@]1([H])C(=O)N(CCCCCN(Cc3cn(CCOCCO[C@@H]4O[C@@H]([C@@H](O[C@H]5O[C@H](COS(O)(=O)=O)[C@@H](O)[C@H](O)[C@H]5NS(O)(=O)=O)[C@H](O)[C@H]4O)C(O)=O)nn3)C(=O)CCC(O)=O)C(=O)[C@@]21[H] |r,c:5| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611977
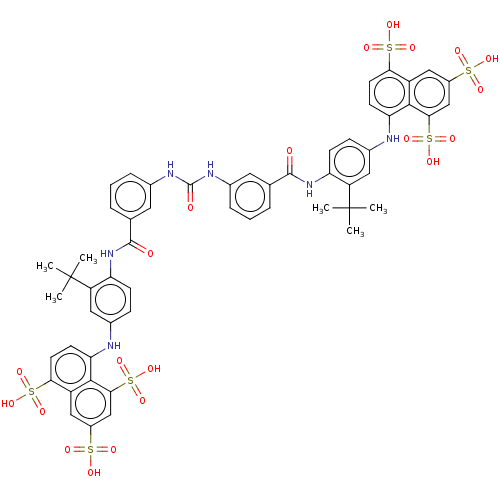
(CHEMBL5288522)Show SMILES [Na;v1].[Na;v1].[Na;v1].[Na;v1].[Na;v1].[Na;v1].[#6]C([#6])([#6])c1cc(-[#7]-c2ccc(c3cc(cc(c23)S([#8])(=O)=O)S([#8])(=O)=O)S([#8])(=O)=O)ccc1-[#7]-[#6](=O)-c1cccc(-[#7]-[#6](=O)-[#7]-c2cccc(c2)-[#6](=O)-[#7]-c2ccc(-[#7]-c3ccc(c4cc(cc(c34)S([#8])(=O)=O)S([#8])(=O)=O)S([#8])(=O)=O)cc2C([#6])([#6])[#6])c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611978
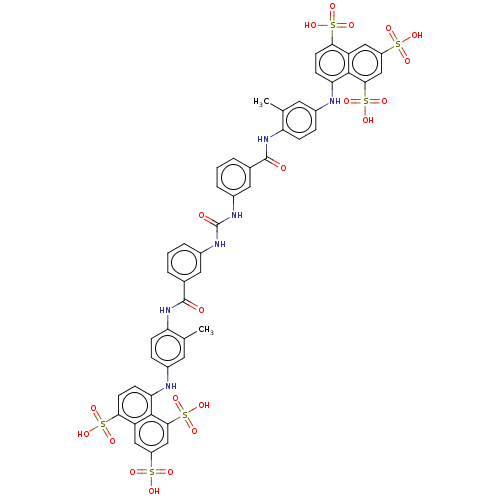
(CHEMBL5268401)Show SMILES [Na;v1].[Na;v1].[Na;v1].[Na;v1].[Na;v1].[Na;v1].[#6]-c1cc(-[#7]-c2ccc(c3cc(cc(c23)S([#8])(=O)=O)S([#8])(=O)=O)S([#8])(=O)=O)ccc1-[#7]-[#6](=O)-c1cccc(-[#7]-[#6](=O)-[#7]-c2cccc(c2)-[#6](=O)-[#7]-c2ccc(-[#7]-c3ccc(c4cc(cc(c34)S([#8])(=O)=O)S([#8])(=O)=O)S([#8])(=O)=O)cc2-[#6])c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611979
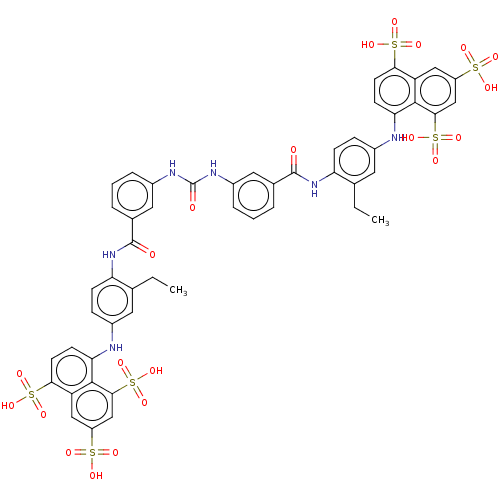
(CHEMBL5268596)Show SMILES [Na;v1].[Na;v1].[Na;v1].[Na;v1].[Na;v1].[Na;v1].[#6]-[#6]-c1cc(-[#7]-c2ccc(c3cc(cc(c23)S([#8])(=O)=O)S([#8])(=O)=O)S([#8])(=O)=O)ccc1-[#7]-[#6](=O)-c1cccc(-[#7]-[#6](=O)-[#7]-c2cccc(c2)-[#6](=O)-[#7]-c2ccc(-[#7]-c3ccc(c4cc(cc(c34)S([#8])(=O)=O)S([#8])(=O)=O)S([#8])(=O)=O)cc2-[#6]-[#6])c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50258216

(CHEMBL4105630)Show SMILES [H][C@@]12CC[C@H](C(=O)Cn3cc(cn3)C#N)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@](C)(O)CC[C@]12[H] |r| Show InChI InChI=1S/C25H35N3O2/c1-24(30)9-7-18-17(11-24)3-4-20-19(18)8-10-25(2)21(20)5-6-22(25)23(29)15-28-14-16(12-26)13-27-28/h13-14,17-22,30H,3-11,15H2,1-2H3/t17-,18+,19-,20-,21+,22-,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sage Therapeutics, Inc. 215 First Street, Cambridge, Massachusetts 02142, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
J Med Chem 60: 7810-7819 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00846
BindingDB Entry DOI: 10.7270/Q2ZK5K4W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50258216

(CHEMBL4105630)Show SMILES [H][C@@]12CC[C@H](C(=O)Cn3cc(cn3)C#N)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@](C)(O)CC[C@]12[H] |r| Show InChI InChI=1S/C25H35N3O2/c1-24(30)9-7-18-17(11-24)3-4-20-19(18)8-10-25(2)21(20)5-6-22(25)23(29)15-28-14-16(12-26)13-27-28/h13-14,17-22,30H,3-11,15H2,1-2H3/t17-,18+,19-,20-,21+,22-,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sage Therapeutics, Inc. 215 First Street, Cambridge, Massachusetts 02142, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 60: 7810-7819 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00846
BindingDB Entry DOI: 10.7270/Q2ZK5K4W |
More data for this
Ligand-Target Pair | |
Inward rectifier potassium channel 2
(Homo sapiens (Human)) | BDBM50258216

(CHEMBL4105630)Show SMILES [H][C@@]12CC[C@H](C(=O)Cn3cc(cn3)C#N)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@](C)(O)CC[C@]12[H] |r| Show InChI InChI=1S/C25H35N3O2/c1-24(30)9-7-18-17(11-24)3-4-20-19(18)8-10-25(2)21(20)5-6-22(25)23(29)15-28-14-16(12-26)13-27-28/h13-14,17-22,30H,3-11,15H2,1-2H3/t17-,18+,19-,20-,21+,22-,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sage Therapeutics, Inc. 215 First Street, Cambridge, Massachusetts 02142, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Kir2.1 (unknown origin) by patch clamp assay |
J Med Chem 60: 7810-7819 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00846
BindingDB Entry DOI: 10.7270/Q2ZK5K4W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50258216

(CHEMBL4105630)Show SMILES [H][C@@]12CC[C@H](C(=O)Cn3cc(cn3)C#N)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@](C)(O)CC[C@]12[H] |r| Show InChI InChI=1S/C25H35N3O2/c1-24(30)9-7-18-17(11-24)3-4-20-19(18)8-10-25(2)21(20)5-6-22(25)23(29)15-28-14-16(12-26)13-27-28/h13-14,17-22,30H,3-11,15H2,1-2H3/t17-,18+,19-,20-,21+,22-,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sage Therapeutics, Inc. 215 First Street, Cambridge, Massachusetts 02142, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 60: 7810-7819 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00846
BindingDB Entry DOI: 10.7270/Q2ZK5K4W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily E/KQT member 1
(Homo sapiens (Human)) | BDBM50258216

(CHEMBL4105630)Show SMILES [H][C@@]12CC[C@H](C(=O)Cn3cc(cn3)C#N)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@](C)(O)CC[C@]12[H] |r| Show InChI InChI=1S/C25H35N3O2/c1-24(30)9-7-18-17(11-24)3-4-20-19(18)8-10-25(2)21(20)5-6-22(25)23(29)15-28-14-16(12-26)13-27-28/h13-14,17-22,30H,3-11,15H2,1-2H3/t17-,18+,19-,20-,21+,22-,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sage Therapeutics, Inc. 215 First Street, Cambridge, Massachusetts 02142, United States.
Curated by ChEMBL
| Assay Description
Inhibition of KCNQ1/minK (unknown origin) by patch clamp assay |
J Med Chem 60: 7810-7819 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00846
BindingDB Entry DOI: 10.7270/Q2ZK5K4W |
More data for this
Ligand-Target Pair | |
Kv channel-interacting protein 2/Potassium voltage-gated channel subfamily D member 3
(Homo sapiens (Human)) | BDBM50258216

(CHEMBL4105630)Show SMILES [H][C@@]12CC[C@H](C(=O)Cn3cc(cn3)C#N)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@](C)(O)CC[C@]12[H] |r| Show InChI InChI=1S/C25H35N3O2/c1-24(30)9-7-18-17(11-24)3-4-20-19(18)8-10-25(2)21(20)5-6-22(25)23(29)15-28-14-16(12-26)13-27-28/h13-14,17-22,30H,3-11,15H2,1-2H3/t17-,18+,19-,20-,21+,22-,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sage Therapeutics, Inc. 215 First Street, Cambridge, Massachusetts 02142, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Kv4.3(unknown origin)/KChIP2.2 (unknown origin) by patch clamp assay |
J Med Chem 60: 7810-7819 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00846
BindingDB Entry DOI: 10.7270/Q2ZK5K4W |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50597430

(DALZANEMDOR | Dalzanemdor)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@](C)(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@](C)(O)C(F)(F)F |r,t:9| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00313
BindingDB Entry DOI: 10.7270/Q2XK8KM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50597430

(DALZANEMDOR | Dalzanemdor)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@](C)(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@](C)(O)C(F)(F)F |r,t:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00313
BindingDB Entry DOI: 10.7270/Q2XK8KM0 |
More data for this
Ligand-Target Pair | |
Voltage-gated potassium channel subunit Kv7.1/Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM50597430

(DALZANEMDOR | Dalzanemdor)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@](C)(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@](C)(O)C(F)(F)F |r,t:9| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00313
BindingDB Entry DOI: 10.7270/Q2XK8KM0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50597430

(DALZANEMDOR | Dalzanemdor)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@](C)(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@](C)(O)C(F)(F)F |r,t:9| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00313
BindingDB Entry DOI: 10.7270/Q2XK8KM0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data