 Found 1622 hits with Last Name = 'sang' and Initial = 'z'
Found 1622 hits with Last Name = 'sang' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50305263
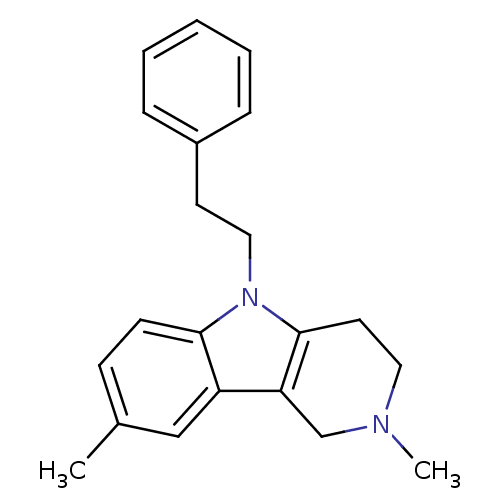
(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231951
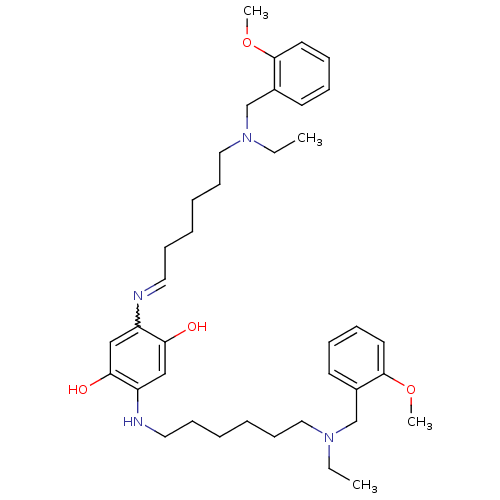
(2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50562062
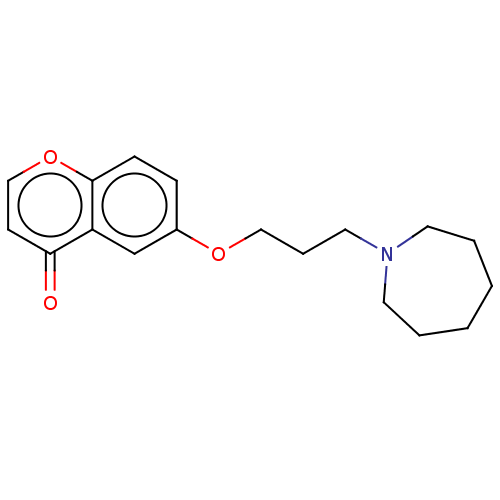
(CHEMBL4754186) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50449388
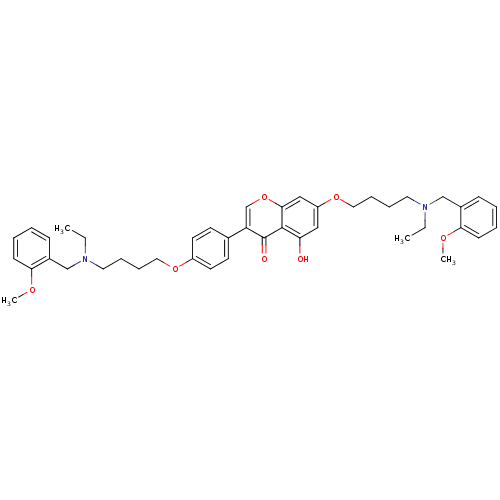
(CHEMBL3127003)Show SMILES CCN(CCCCOc1ccc(cc1)-c1coc2cc(OCCCCN(CC)Cc3ccccc3OC)cc(O)c2c1=O)Cc1ccccc1OC Show InChI InChI=1S/C43H52N2O7/c1-5-44(29-33-15-7-9-17-39(33)48-3)23-11-13-25-50-35-21-19-32(20-22-35)37-31-52-41-28-36(27-38(46)42(41)43(37)47)51-26-14-12-24-45(6-2)30-34-16-8-10-18-40(34)49-4/h7-10,15-22,27-28,31,46H,5-6,11-14,23-26,29-30H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AchE using acetylthiocholine as substrate after 15 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 76: 314-31 (2014)
Article DOI: 10.1016/j.ejmech.2014.02.045
BindingDB Entry DOI: 10.7270/Q20R9QXD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50564217
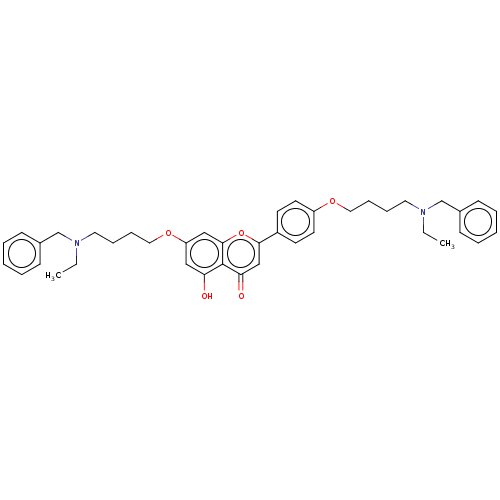
(CHEMBL4783427)Show SMILES CCN(CCCCOc1ccc(cc1)-c1cc(=O)c2c(O)cc(OCCCCN(CC)Cc3ccccc3)cc2o1)Cc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 394 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human AChE using acetylthiocholine as substrate by Line-weaver Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112180
BindingDB Entry DOI: 10.7270/Q2N301PX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50569012
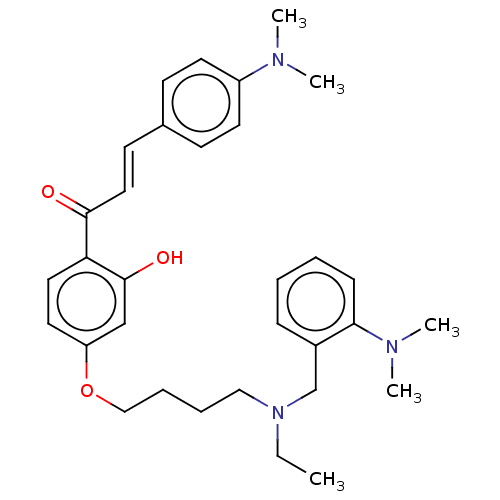
(CHEMBL4865817)Show SMILES CCN(CCCCOc1ccc(C(=O)\C=C\c2ccc(cc2)N(C)C)c(O)c1)Cc1ccccc1N(C)C | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of electric eel AChE by reciprocal Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113310
BindingDB Entry DOI: 10.7270/Q28G8QGX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585545

(CHEMBL5074018)Show SMILES NC(=O)C1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585540

(CHEMBL5091789)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585541

(CHEMBL5071942)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585552

(CHEMBL5076528)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585560

(CHEMBL5071085)Show SMILES C[C@H](NC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOC[C@@H]2C)N2CCOCC2)cc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585553
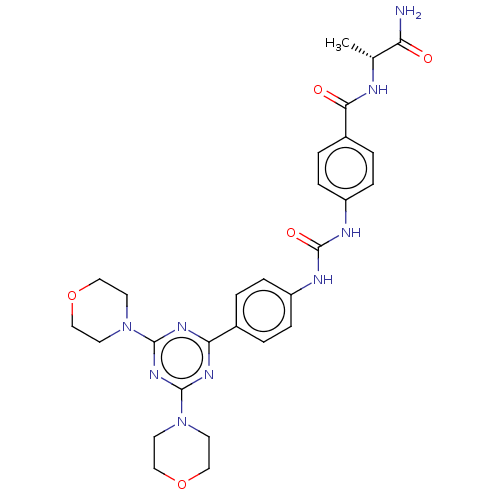
(CHEMBL5093540)Show SMILES C[C@@H](NC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585558
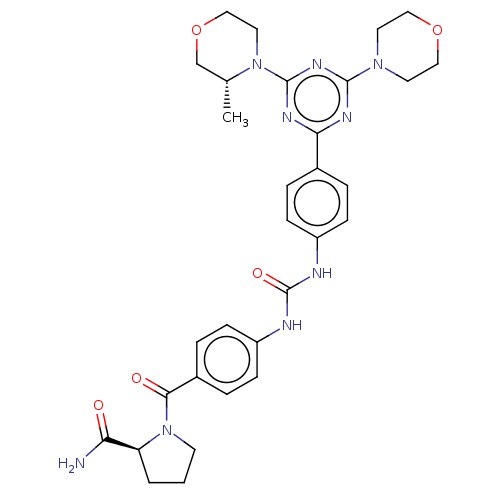
(CHEMBL5080996)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC[C@H]2C(N)=O)cc1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585559
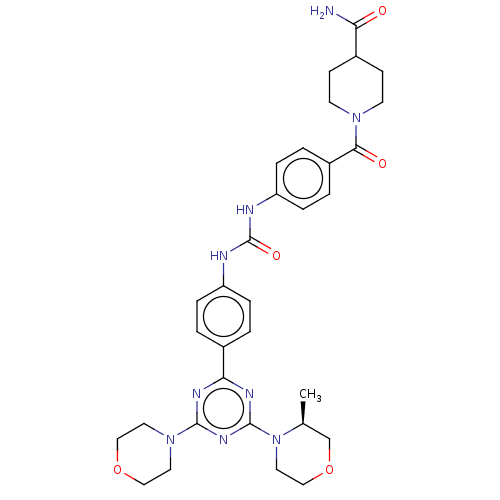
(CHEMBL5085872)Show SMILES C[C@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC(CC2)C(N)=O)cc1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585545

(CHEMBL5074018)Show SMILES NC(=O)C1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585550
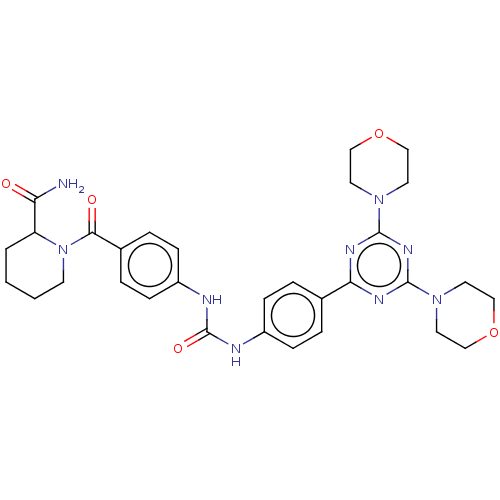
(CHEMBL5079887)Show SMILES NC(=O)C1CCCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585551
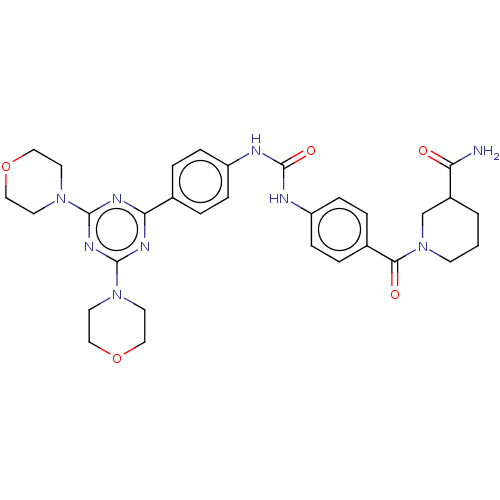
(CHEMBL5091543)Show SMILES NC(=O)C1CCCN(C1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585558

(CHEMBL5080996)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC[C@H]2C(N)=O)cc1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585552

(CHEMBL5076528)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585544

(CHEMBL5083002)Show SMILES [H][C@@]12C[C@]1([H])N([C@@H](C2)C(N)=O)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585544

(CHEMBL5083002)Show SMILES [H][C@@]12C[C@]1([H])N([C@@H](C2)C(N)=O)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585553

(CHEMBL5093540)Show SMILES C[C@@H](NC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308135
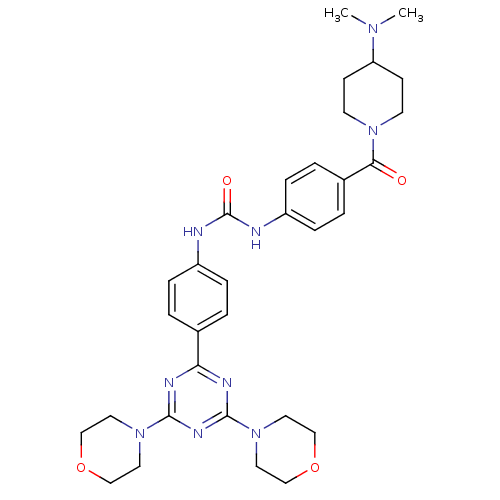
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585551

(CHEMBL5091543)Show SMILES NC(=O)C1CCCN(C1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585547

(CHEMBL5079231)Show SMILES CN(C)[C@@H]1C[C@H](N(C1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-B |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112265
BindingDB Entry DOI: 10.7270/Q2W099J0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585549

(CHEMBL5084675)Show SMILES NC(=O)[C@@H]1CC(O)CN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585540

(CHEMBL5091789)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50207456

(CHEMBL3943883)Show SMILES COc1cc2OC\C(=C/c3ccc(CN4CCCCC4)c(O)c3)C(=O)c2cc1OC Show InChI InChI=1S/C24H27NO5/c1-28-22-12-19-21(13-23(22)29-2)30-15-18(24(19)27)10-16-6-7-17(20(26)11-16)14-25-8-4-3-5-9-25/h6-7,10-13,26H,3-5,8-9,14-15H2,1-2H3/b18-10+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method |
Bioorg Med Chem 25: 714-726 (2017)
Article DOI: 10.1016/j.bmc.2016.11.048
BindingDB Entry DOI: 10.7270/Q21C1ZVW |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-A using kynuramine as substrate measured after 30 mins by fluorescence assay |
Bioorg Med Chem 25: 714-726 (2017)
Article DOI: 10.1016/j.bmc.2016.11.048
BindingDB Entry DOI: 10.7270/Q21C1ZVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-A by multimode plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113310
BindingDB Entry DOI: 10.7270/Q28G8QGX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected High 5 insect cells using kynuramine as substrate incubated for 30 mins by sp... |
Bioorg Med Chem 25: 1030-1041 (2017)
Article DOI: 10.1016/j.bmc.2016.12.013
BindingDB Entry DOI: 10.7270/Q2PR7XZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50199101

(CHEMBL3920226)Show InChI InChI=1S/C15H13FO3/c1-10(17)14-6-5-13(8-15(14)18)19-9-11-3-2-4-12(16)7-11/h2-8,18H,9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang 473061, China. Electronic address: sangzhipei@126.com.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-B expressed in insect cell microsomes using kynuramine as substrate by fluorescence spectrophotometric method |
Bioorg Med Chem Lett 27: 5046-5052 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.057
BindingDB Entry DOI: 10.7270/Q2XS5XTR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585550

(CHEMBL5079887)Show SMILES NC(=O)C1CCCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585548
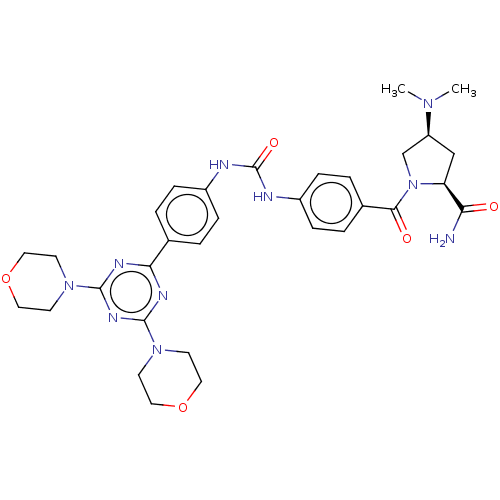
(CHEMBL5071429)Show SMILES CN(C)[C@H]1C[C@H](N(C1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50585540

(CHEMBL5091789)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585541

(CHEMBL5071942)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585546
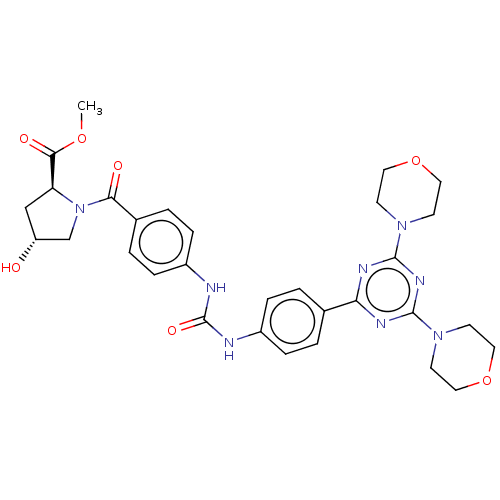
(CHEMBL5089561)Show SMILES COC(=O)[C@@H]1C[C@@H](O)CN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang 473061, China. Electronic address: sangzhipei@126.com.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-A using kynuramine as substrate after 30 mins by fluorescence method |
Bioorg Med Chem Lett 27: 5046-5052 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.057
BindingDB Entry DOI: 10.7270/Q2XS5XTR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang 473061, China. Electronic address: sangzhipei@126.com.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using kynuramine as substrate after 30 mins by fluorescence ... |
Bioorg Med Chem 25: 3006-3017 (2017)
Article DOI: 10.1016/j.bmc.2017.03.070
BindingDB Entry DOI: 10.7270/Q2F76G04 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585542
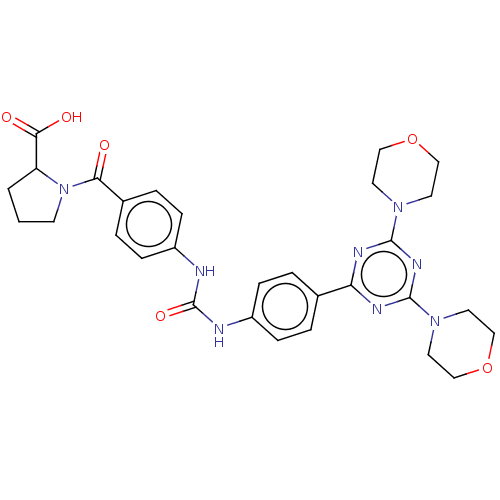
(CHEMBL5094579)Show SMILES OC(=O)C1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585543
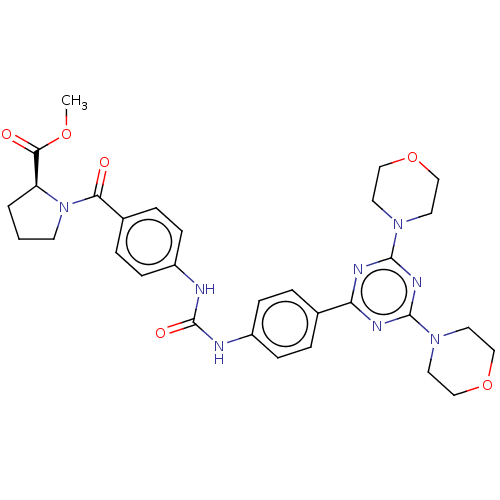
(CHEMBL5086369)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585562
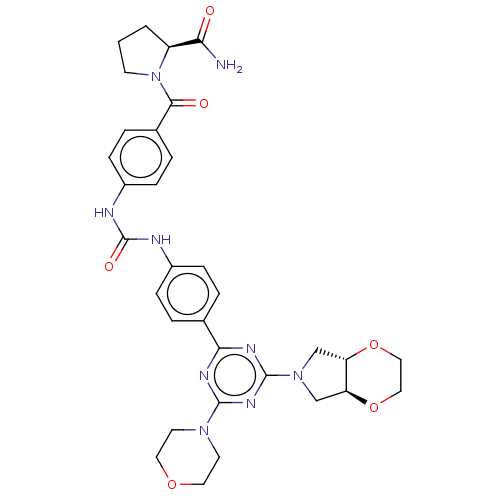
(CHEMBL5078347)Show SMILES [H][C@]12CN(C[C@]1([H])OCCO2)c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC[C@H]2C(N)=O)cc1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50585541

(CHEMBL5071942)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50585559

(CHEMBL5085872)Show SMILES C[C@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC(CC2)C(N)=O)cc1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate in presence of ATP incubated for 60 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50585561
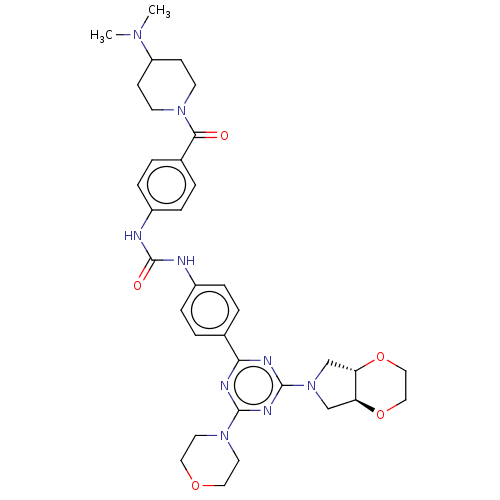
(CHEMBL5079397)Show SMILES [H][C@]12CN(C[C@]1([H])OCCO2)c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC(CC2)N(C)C)cc1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in presence of ATP incubated for 45 mins by Lance ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114055
BindingDB Entry DOI: 10.7270/Q2NV9P4M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data