 Found 293 hits with Last Name = 'ni' and Initial = 'zj'
Found 293 hits with Last Name = 'ni' and Initial = 'zj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM349368

(US10207991, Ex. Cpd. No. 11 | US10611728, Example ...)Show SMILES COc1cc2CN(Cc2cc1OC)[C@H](C)CCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H23Cl2NO2/c1-13(4-5-14-6-7-17(21)18(22)8-14)23-11-15-9-19(24-2)20(25-3)10-16(15)12-23/h6-10,13H,4-5,11-12H2,1-3H3/t13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573588
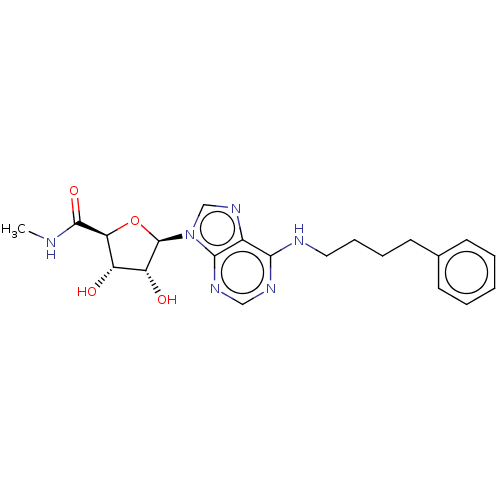
(CHEMBL4871554)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573589
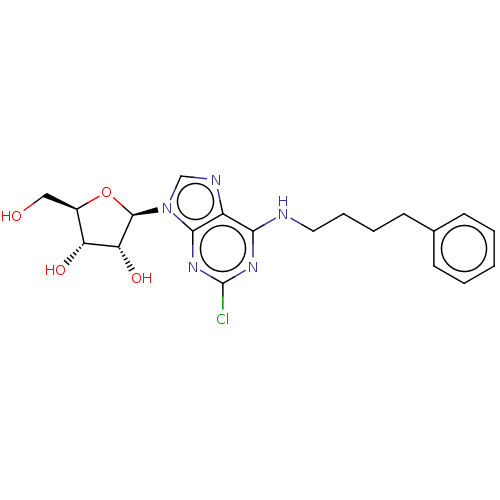
(CHEMBL4864883)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)nc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM349547
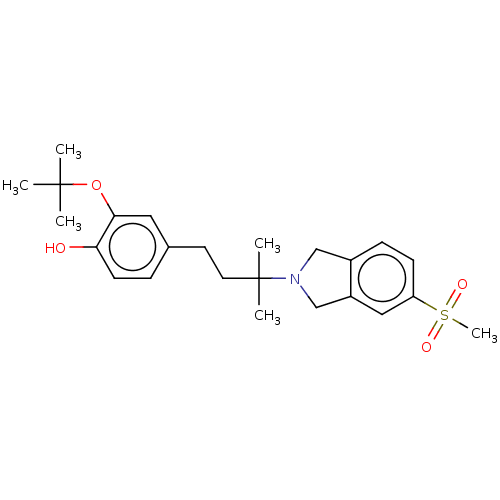
(US10207991, Ex. Cpd. No. 62 | US10611728, Example ...)Show SMILES CC(C)(C)Oc1cc(CCC(C)(C)N2Cc3ccc(cc3C2)S(C)(=O)=O)ccc1O Show InChI InChI=1S/C24H33NO4S/c1-23(2,3)29-22-13-17(7-10-21(22)26)11-12-24(4,5)25-15-18-8-9-20(30(6,27)28)14-19(18)16-25/h7-10,13-14,26H,11-12,15-16H2,1-6H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50577481
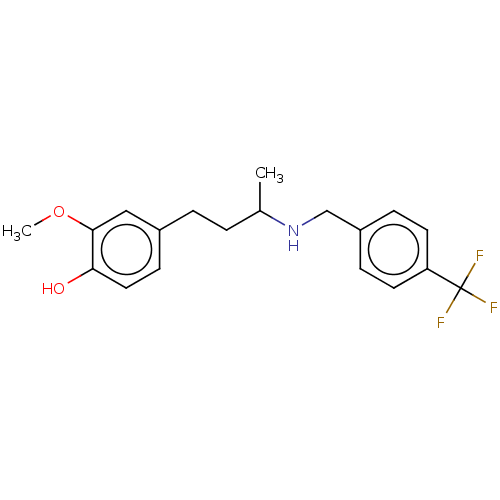
(CHEMBL4865307) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573587

(CHEMBL4861092)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM349513
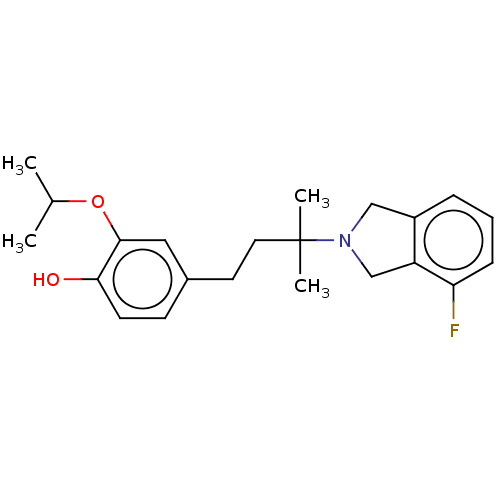
(US10207991, Ex. Cpd. No. 28 | US10611728, Example ...)Show InChI InChI=1S/C22H28FNO2/c1-15(2)26-21-12-16(8-9-20(21)25)10-11-22(3,4)24-13-17-6-5-7-19(23)18(17)14-24/h5-9,12,15,25H,10-11,13-14H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50452336
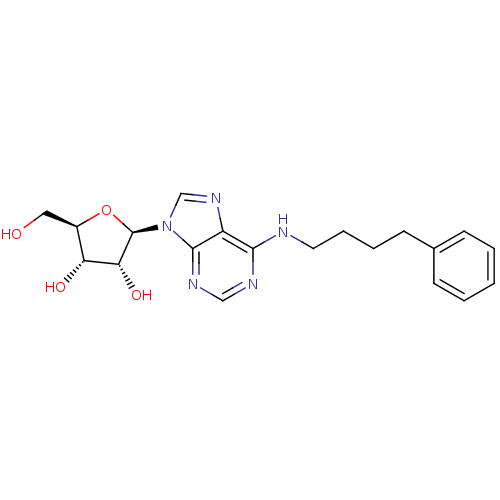
(CHEMBL2113406)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C20H25N5O4/c26-10-14-16(27)17(28)20(29-14)25-12-24-15-18(22-11-23-19(15)25)21-9-5-4-8-13-6-2-1-3-7-13/h1-3,6-7,11-12,14,16-17,20,26-28H,4-5,8-10H2,(H,21,22,23)/t14-,16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50205709
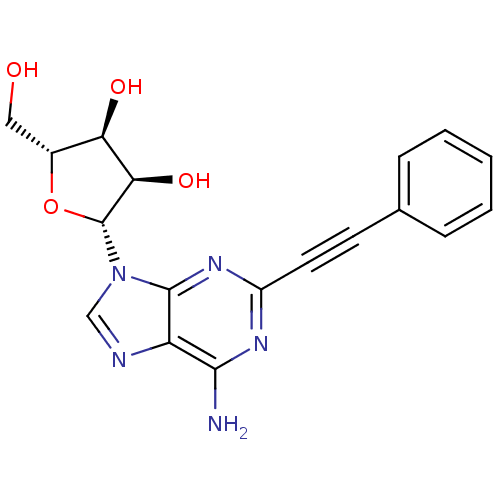
((2R,3R,4S,5R)-2-(6-amino-2-(phenylethynyl)-9H-puri...)Show SMILES Nc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C#Cc1ccccc1 Show InChI InChI=1S/C18H17N5O4/c19-16-13-17(22-12(21-16)7-6-10-4-2-1-3-5-10)23(9-20-13)18-15(26)14(25)11(8-24)27-18/h1-5,9,11,14-15,18,24-26H,8H2,(H2,19,21,22)/t11-,14-,15-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50577482

(CHEMBL4848550)Show SMILES C[C@H](CCc1ccc(Cl)c(Cl)c1)NCc1ccc(cc1)C(F)(F)F |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50577484

(CHEMBL4863873)Show SMILES C[C@H](CCc1ccc(Cl)cc1)NCc1ccc(cc1)C(F)(F)F |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50452335
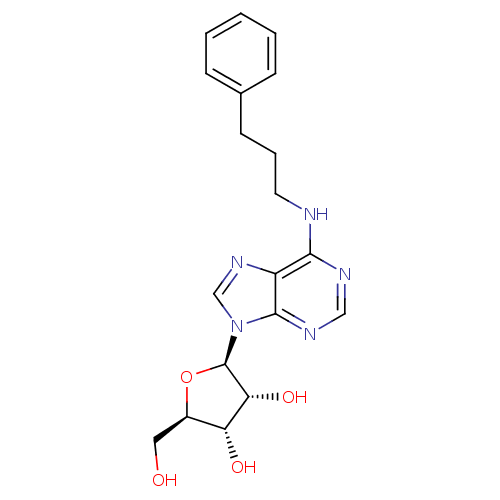
(CHEMBL2113484)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C19H23N5O4/c25-9-13-15(26)16(27)19(28-13)24-11-23-14-17(21-10-22-18(14)24)20-8-4-7-12-5-2-1-3-6-12/h1-3,5-6,10-11,13,15-16,19,25-27H,4,7-9H2,(H,20,21,22)/t13-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM349324

(US10207991, Ex. Cpd. No. 8 | US10611728, Example C...)Show SMILES COc1cc(CC[C@@H](C)N2Cc3cccc(F)c3C2)ccc1O |r| Show InChI InChI=1S/C19H22FNO2/c1-13(6-7-14-8-9-18(22)19(10-14)23-2)21-11-15-4-3-5-17(20)16(15)12-21/h3-5,8-10,13,22H,6-7,11-12H2,1-2H3/t13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50452335

(CHEMBL2113484)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C19H23N5O4/c25-9-13-15(26)16(27)19(28-13)24-11-23-14-17(21-10-22-18(14)24)20-8-4-7-12-5-2-1-3-6-12/h1-3,5-6,10-11,13,15-16,19,25-27H,4,7-9H2,(H,20,21,22)/t13-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50577483

(CHEMBL4869437)Show SMILES C[C@@H](CCc1ccc(Cl)c(Cl)c1)NCc1ccc(cc1)C(F)(F)F |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Sigma 2-receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00048
BindingDB Entry DOI: 10.7270/Q21C21Q9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50205709

((2R,3R,4S,5R)-2-(6-amino-2-(phenylethynyl)-9H-puri...)Show SMILES Nc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C#Cc1ccccc1 Show InChI InChI=1S/C18H17N5O4/c19-16-13-17(22-12(21-16)7-6-10-4-2-1-3-5-10)23(9-20-13)18-15(26)14(25)11(8-24)27-18/h1-5,9,11,14-15,18,24-26H,8H2,(H2,19,21,22)/t11-,14-,15-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50573587

(CHEMBL4861092)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 803 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50366687
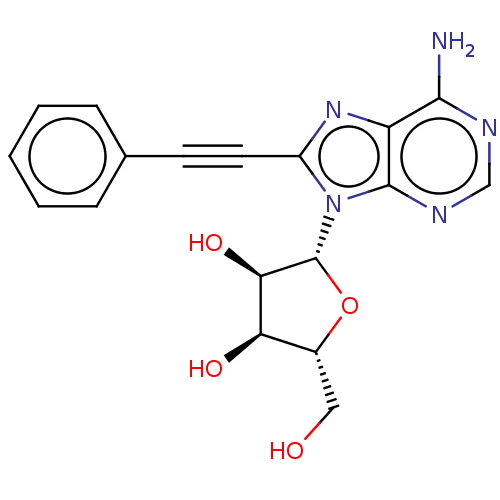
(CHEMBL3350497 | CHEMBL611516)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(nc12)C#Cc1ccccc1 |r| Show InChI InChI=1S/C18H17N5O4/c19-16-13-17(21-9-20-16)23(18-15(26)14(25)11(8-24)27-18)12(22-13)7-6-10-4-2-1-3-5-10/h1-5,9,11,14-15,18,24-26H,8H2,(H2,19,20,21)/t11-,14-,15-,18?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 856 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50452336

(CHEMBL2113406)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C20H25N5O4/c26-10-14-16(27)17(28)20(29-14)25-12-24-15-18(22-11-23-19(15)25)21-9-5-4-8-13-6-2-1-3-7-13/h1-3,6-7,11-12,14,16-17,20,26-28H,4-5,8-10H2,(H,21,22,23)/t14-,16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 864 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573591
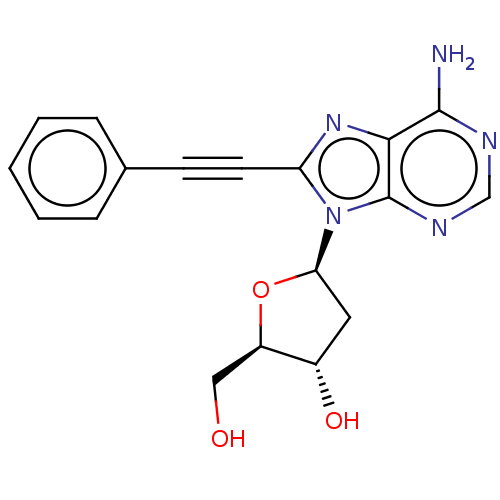
(CHEMBL4847237)Show SMILES Nc1ncnc2n([C@H]3C[C@H](O)[C@@H](CO)O3)c(nc12)C#Cc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 879 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50205709

((2R,3R,4S,5R)-2-(6-amino-2-(phenylethynyl)-9H-puri...)Show SMILES Nc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C#Cc1ccccc1 Show InChI InChI=1S/C18H17N5O4/c19-16-13-17(22-12(21-16)7-6-10-4-2-1-3-5-10)23(9-20-13)18-15(26)14(25)11(8-24)27-18/h1-5,9,11,14-15,18,24-26H,8H2,(H2,19,21,22)/t11-,14-,15-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HeLa cell membrane incubated for 30 mins by microplate beta scintillation... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573593
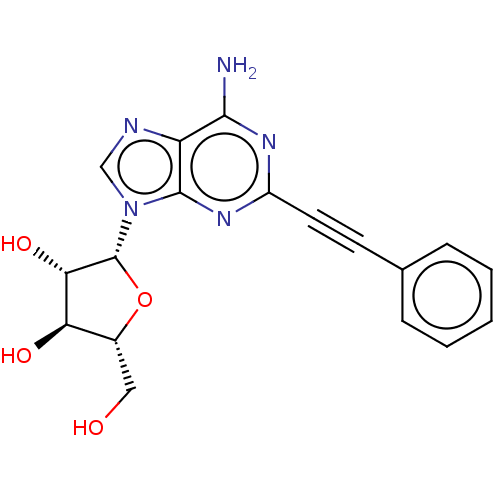
(CHEMBL4855141)Show SMILES Nc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O)C#Cc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50573588

(CHEMBL4871554)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK cell membrane incubated for 30 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50573592
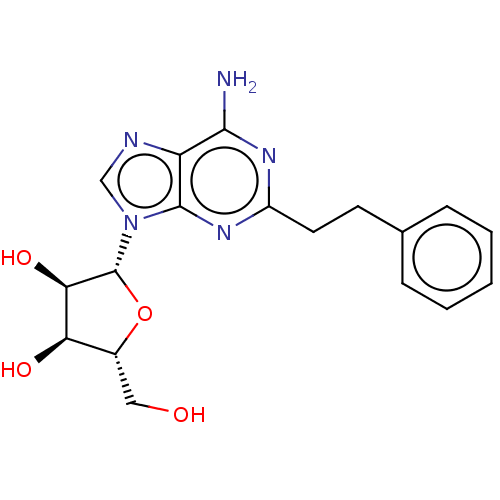
(CHEMBL4852007)Show SMILES Nc1nc(CCc2ccccc2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50573591

(CHEMBL4847237)Show SMILES Nc1ncnc2n([C@H]3C[C@H](O)[C@@H](CO)O3)c(nc12)C#Cc1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HeLa cell membrane incubated for 30 mins by microplate beta scintillation... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50452335

(CHEMBL2113484)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C19H23N5O4/c25-9-13-15(26)16(27)19(28-13)24-11-23-14-17(21-10-22-18(14)24)20-8-4-7-12-5-2-1-3-6-12/h1-3,5-6,10-11,13,15-16,19,25-27H,4,7-9H2,(H,20,21,22)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HeLa cell membrane incubated for 30 mins by microplate beta scintillation... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50573587

(CHEMBL4861092)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK cell membrane incubated for 30 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50573589

(CHEMBL4864883)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)nc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50573587

(CHEMBL4861092)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HeLa cell membrane incubated for 30 mins by microplate beta scintillation... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573592

(CHEMBL4852007)Show SMILES Nc1nc(CCc2ccccc2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50573590
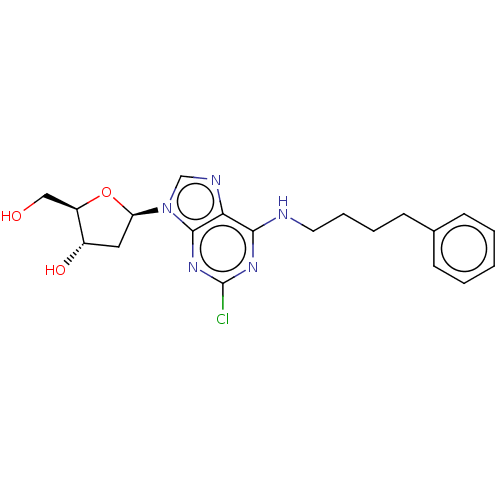
(CHEMBL4873585)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)nc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cell membrane incubated for 180 mins by microplate beta scintillation cou... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50573590

(CHEMBL4873585)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(NCCCCc3ccccc3)nc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membrane incubated for 60 mins by microplate beta scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113607
BindingDB Entry DOI: 10.7270/Q2JD51KN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452149
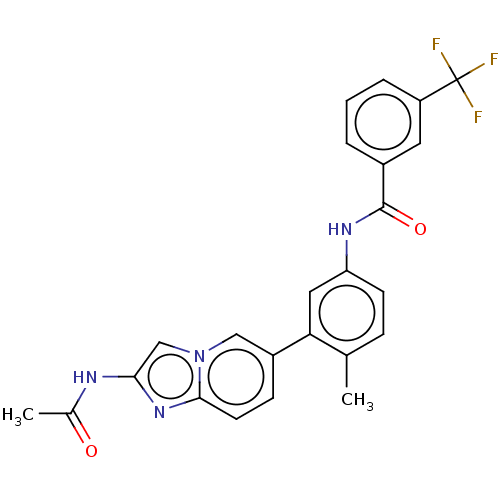
(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452150
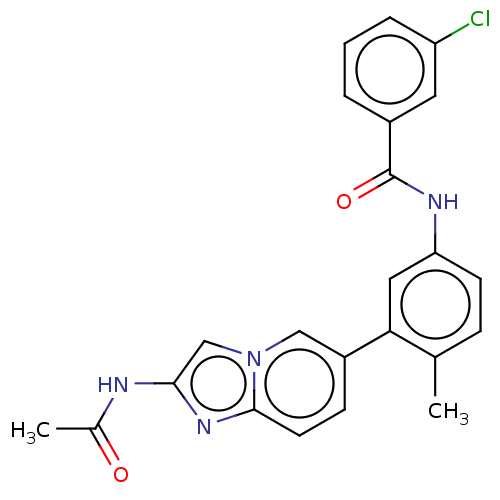
(CHEMBL4216386)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(Cl)c2)ccc1C Show InChI InChI=1S/C23H19ClN4O2/c1-14-6-8-19(26-23(30)16-4-3-5-18(24)10-16)11-20(14)17-7-9-22-27-21(25-15(2)29)13-28(22)12-17/h3-13H,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452152
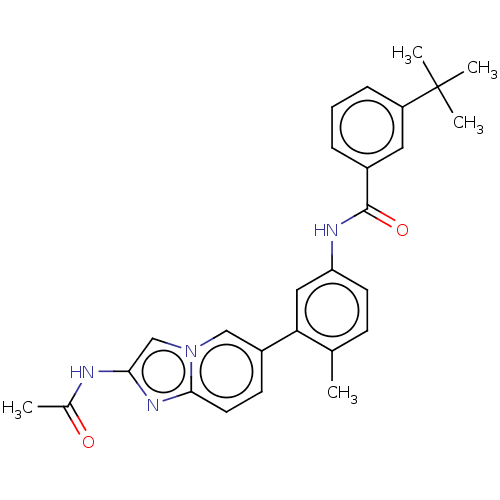
(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185219
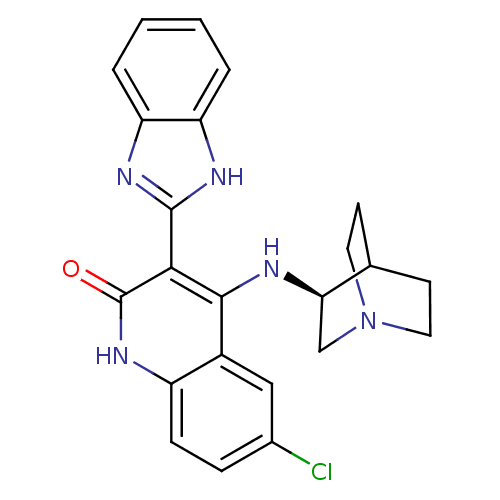
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(quinu...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CN4CCC3CC4)c2c1 |wU:20.21,TLB:19:20:24.23:26.27,(4.27,-32.71,;5.6,-33.48,;5.6,-35.02,;6.93,-35.79,;8.27,-35.03,;9.61,-35.79,;10.95,-35.01,;12.28,-35.78,;10.94,-33.46,;12.27,-32.69,;13.68,-33.32,;14.7,-32.18,;16.24,-32.18,;17.02,-30.84,;16.23,-29.5,;14.69,-29.51,;13.93,-30.84,;12.43,-31.16,;9.59,-32.69,;9.59,-31.15,;8.64,-29.94,;8.36,-28.54,;7.01,-27.94,;5.55,-28.58,;5.74,-29.96,;7.27,-29.3,;7.53,-27.41,;7.08,-26.31,;8.26,-33.47,;6.93,-32.71,)| Show InChI InChI=1S/C23H22ClN5O/c24-14-5-6-16-15(11-14)21(25-19-12-29-9-7-13(19)8-10-29)20(23(30)28-16)22-26-17-3-1-2-4-18(17)27-22/h1-6,11,13,19H,7-10,12H2,(H,26,27)(H2,25,28,30)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185221
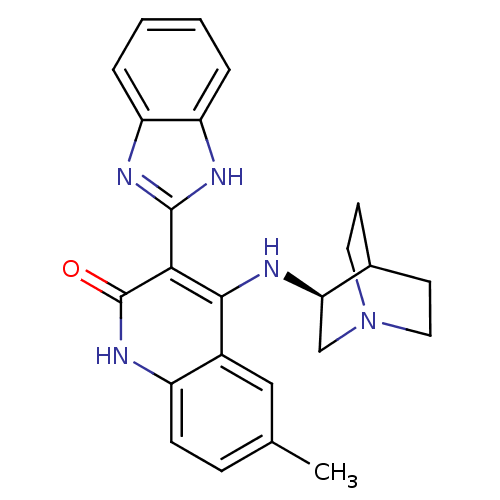
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-methyl-4-(quinu...)Show SMILES Cc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CN4CCC3CC4)c2c1 |wU:20.21,TLB:19:20:24.23:26.27,(17.38,-9.04,;18.71,-9.81,;18.71,-11.36,;20.04,-12.13,;21.38,-11.36,;22.72,-12.13,;24.06,-11.35,;25.39,-12.12,;24.05,-9.8,;25.38,-9.02,;26.79,-9.65,;27.81,-8.51,;29.35,-8.51,;30.13,-7.17,;29.34,-5.83,;27.8,-5.84,;27.04,-7.17,;25.54,-7.5,;22.7,-9.02,;22.7,-7.48,;21.75,-6.27,;21.47,-4.88,;20.12,-4.28,;18.65,-4.92,;18.85,-6.3,;20.38,-5.64,;20.64,-3.75,;20.19,-2.64,;21.37,-9.81,;20.04,-9.04,)| Show InChI InChI=1S/C24H25N5O/c1-14-6-7-17-16(12-14)22(25-20-13-29-10-8-15(20)9-11-29)21(24(30)28-17)23-26-18-4-2-3-5-19(18)27-23/h2-7,12,15,20H,8-11,13H2,1H3,(H,26,27)(H2,25,28,30)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185218
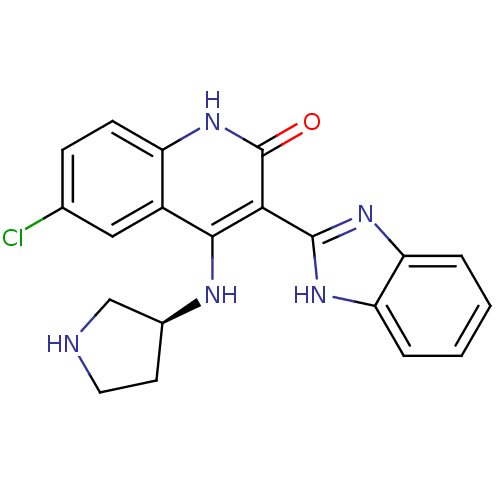
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(pyrro...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@H]3CCNC3)c2c1 Show InChI InChI=1S/C20H18ClN5O/c21-11-5-6-14-13(9-11)18(23-12-7-8-22-10-12)17(20(27)26-14)19-24-15-3-1-2-4-16(15)25-19/h1-6,9,12,22H,7-8,10H2,(H,24,25)(H2,23,26,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313013
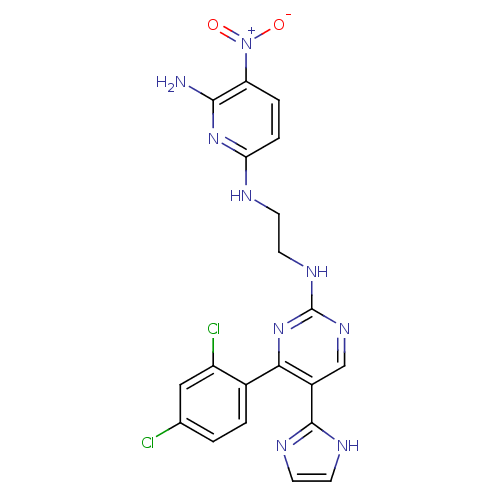
(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50243389
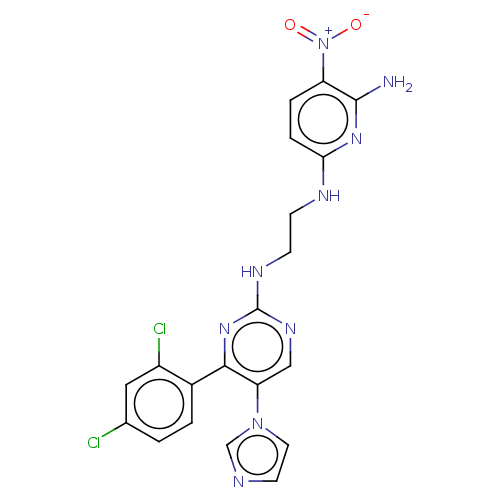
(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452147
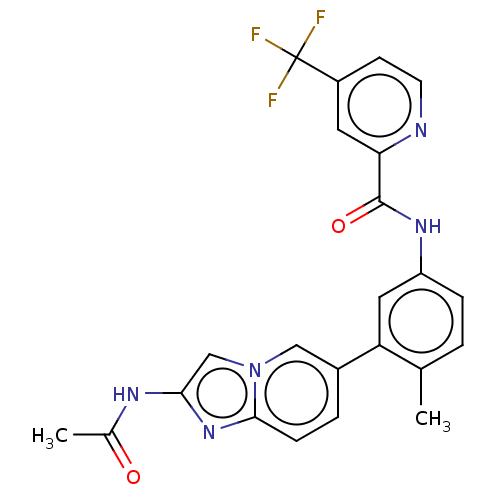
(CHEMBL4204192)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cc(ccn2)C(F)(F)F)ccc1C Show InChI InChI=1S/C23H18F3N5O2/c1-13-3-5-17(29-22(33)19-9-16(7-8-27-19)23(24,25)26)10-18(13)15-4-6-21-30-20(28-14(2)32)12-31(21)11-15/h3-12H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185217
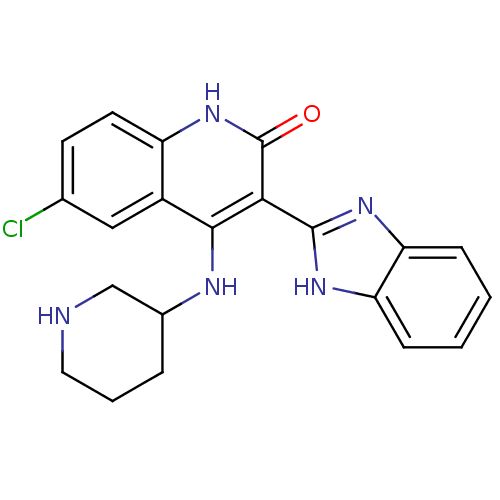
(3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(piperidin...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(NC3CCCNC3)c2c1 Show InChI InChI=1S/C21H20ClN5O/c22-12-7-8-15-14(10-12)19(24-13-4-3-9-23-11-13)18(21(28)27-15)20-25-16-5-1-2-6-17(16)26-20/h1-2,5-8,10,13,23H,3-4,9,11H2,(H,25,26)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185224
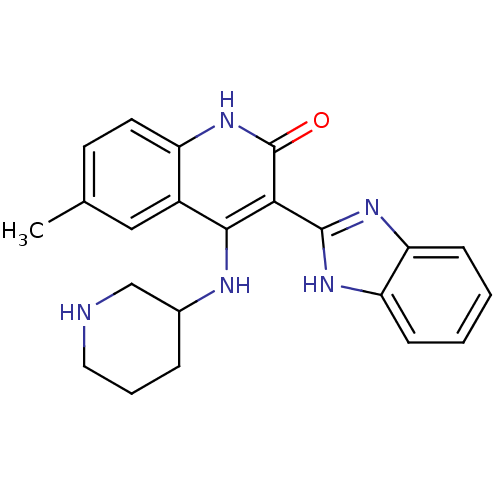
(3-(1H-benzo[d]imidazol-2-yl)-6-methyl-4-(piperidin...)Show SMILES Cc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(NC3CCCNC3)c2c1 Show InChI InChI=1S/C22H23N5O/c1-13-8-9-16-15(11-13)20(24-14-5-4-10-23-12-14)19(22(28)27-16)21-25-17-6-2-3-7-18(17)26-21/h2-3,6-9,11,14,23H,4-5,10,12H2,1H3,(H,25,26)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452152

(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452151
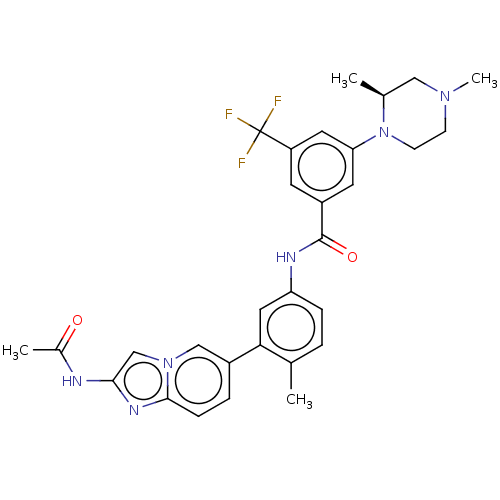
(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140266
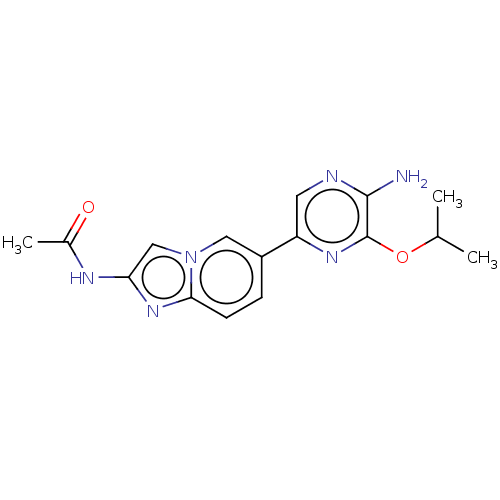
(CHEMBL3753366)Show InChI InChI=1S/C16H18N6O2/c1-9(2)24-16-15(17)18-6-12(20-16)11-4-5-14-21-13(19-10(3)23)8-22(14)7-11/h4-9H,1-3H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721
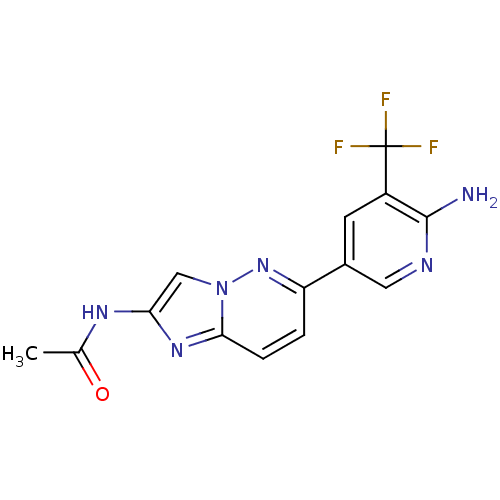
(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721

(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380371
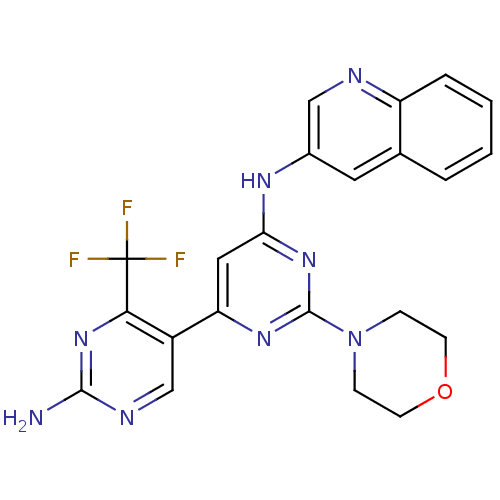
(CHEMBL2017970)Show SMILES Nc1ncc(-c2cc(Nc3cnc4ccccc4c3)nc(n2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C22H19F3N8O/c23-22(24,25)19-15(12-28-20(26)32-19)17-10-18(31-21(30-17)33-5-7-34-8-6-33)29-14-9-13-3-1-2-4-16(13)27-11-14/h1-4,9-12H,5-8H2,(H2,26,28,32)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
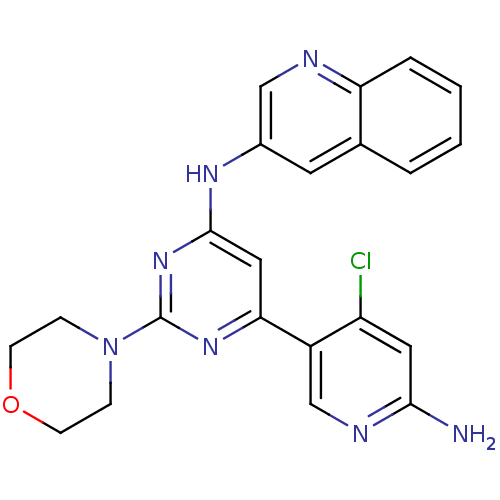
(Homo sapiens (Human)) | BDBM50380366
(CHEMBL2017965)Show SMILES Nc1cc(Cl)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H20ClN7O/c23-17-10-20(24)26-13-16(17)19-11-21(29-22(28-19)30-5-7-31-8-6-30)27-15-9-14-3-1-2-4-18(14)25-12-15/h1-4,9-13H,5-8H2,(H2,24,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data