 Found 180 hits with Last Name = 'wang' and Initial = 'zx'
Found 180 hits with Last Name = 'wang' and Initial = 'zx' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM50033531
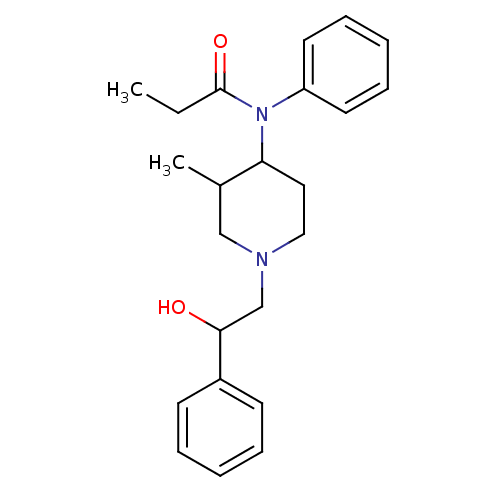
(CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...)Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033533

(CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033534
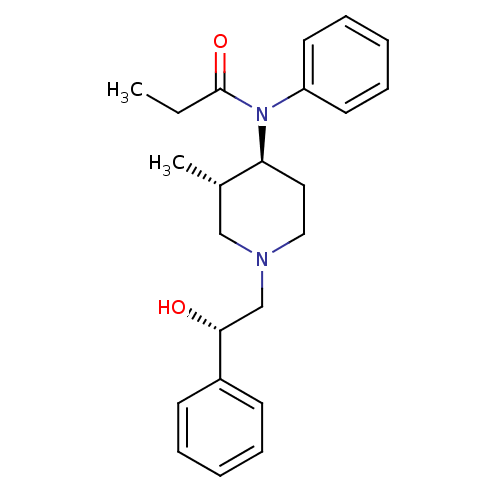
(CHEMBL338510 | N-[(3S,4S)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033536

(CHEMBL121494 | N-[(3R,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033530
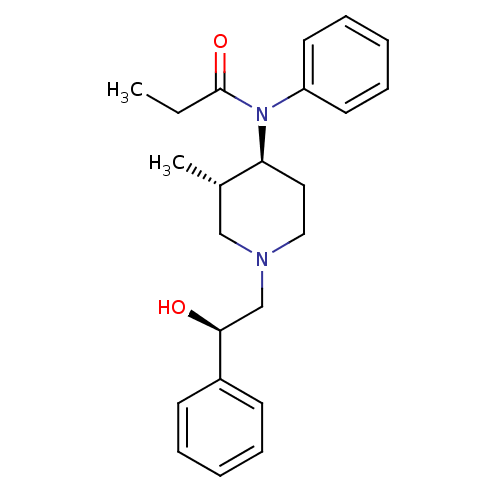
(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033530

(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033537
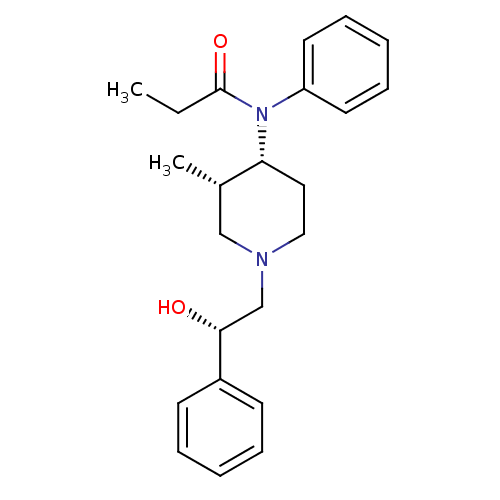
(CHEMBL121060 | N-[(3S,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033532

(CHEMBL435380 | N-[(3S,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged Aurora B (1 to 403 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033531

(CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...)Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033536

(CHEMBL121494 | N-[(3R,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor delta 1 using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033533

(CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033530

(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033530

(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033537

(CHEMBL121060 | N-[(3S,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033534

(CHEMBL338510 | N-[(3S,4S)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50033532

(CHEMBL435380 | N-[(3S,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against delta-opioid receptor using [3H]-DPDPE radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50466808

(AZD-1152-HQPA | Barasertib)Show SMILES CCN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)[nH]n3)ncnc2c1 Show InChI InChI=1S/C26H30FN7O3/c1-2-34(10-11-35)9-4-12-37-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(36)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17,35H,2,4,9-12,15H2,1H3,(H,30,36)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570288
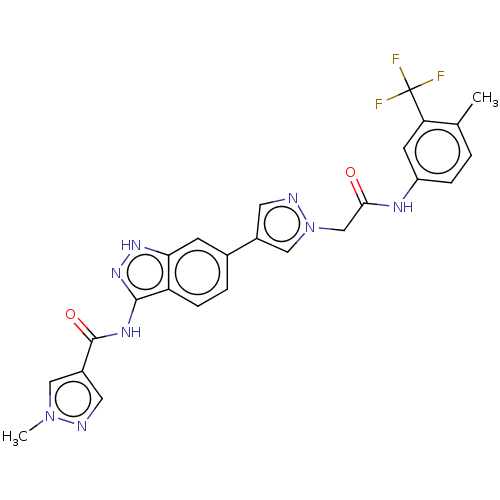
(CHEMBL4878552)Show SMILES Cc1ccc(NC(=O)Cn2cc(cn2)-c2ccc3c(NC(=O)c4cnn(C)c4)n[nH]c3c2)cc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) after 1 hr in presence of ATP by Kinase-Glo reagent-based luminescence assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126885
BindingDB Entry DOI: 10.7270/Q27P92X1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570281
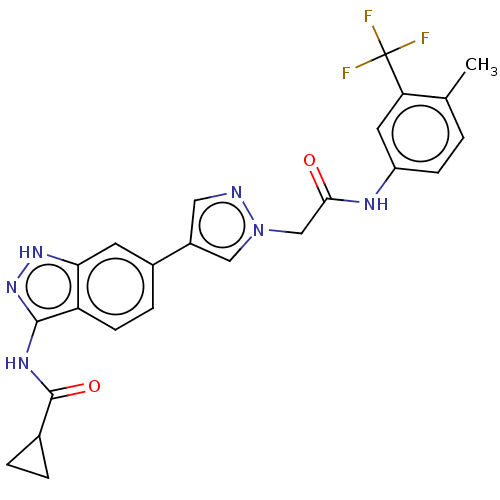
(CHEMBL4858003)Show SMILES Cc1ccc(NC(=O)Cn2cc(cn2)-c2ccc3c(NC(=O)C4CC4)n[nH]c3c2)cc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570283
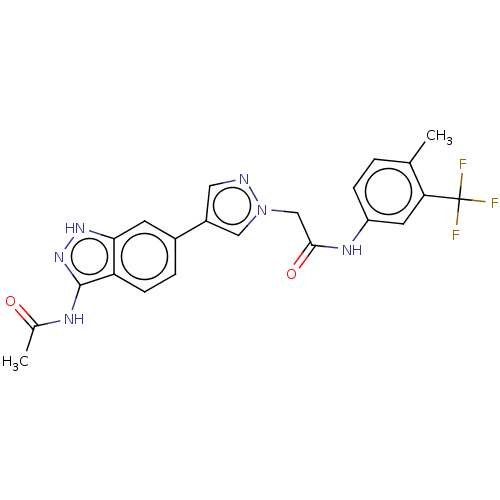
(CHEMBL4870465)Show SMILES CC(=O)Nc1n[nH]c2cc(ccc12)-c1cnn(CC(=O)Nc2ccc(C)c(c2)C(F)(F)F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570287
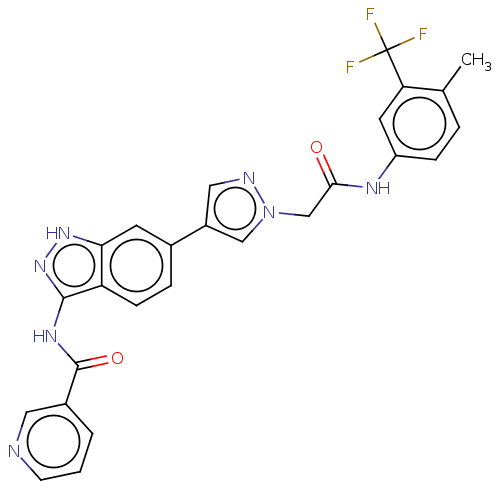
(CHEMBL4872941)Show SMILES Cc1ccc(NC(=O)Cn2cc(cn2)-c2ccc3c(NC(=O)c4cccnc4)n[nH]c3c2)cc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570278
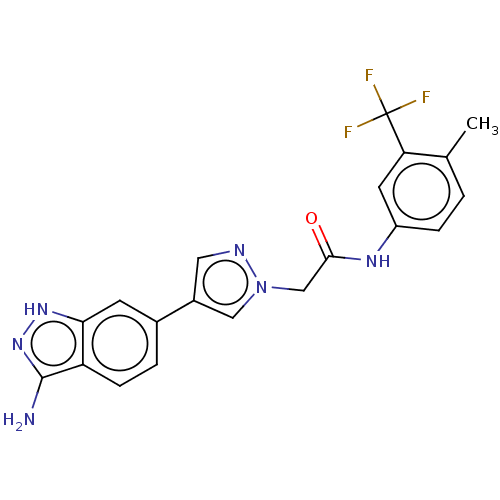
(CHEMBL4853592)Show SMILES Cc1ccc(NC(=O)Cn2cc(cn2)-c2ccc3c(N)n[nH]c3c2)cc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50202356

(CHEMBL218806 | cyclo(-D-Tyr-D-MeArg-L-Arg-L-Nal-Gl...)Show SMILES [#6]-[#7]-1-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc3ccccc3c2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O |r| Show InChI InChI=1S/C37H49N11O6/c1-48-30(9-5-17-43-37(40)41)34(53)47-28(19-22-11-14-26(49)15-12-22)32(51)44-21-31(50)45-29(20-23-10-13-24-6-2-3-7-25(24)18-23)33(52)46-27(35(48)54)8-4-16-42-36(38)39/h2-3,6-7,10-15,18,27-30,49H,4-5,8-9,16-17,19-21H2,1H3,(H,44,51)(H,45,50)(H,46,52)(H,47,53)(H4,38,39,42)(H4,40,41,43)/t27-,28+,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50202350
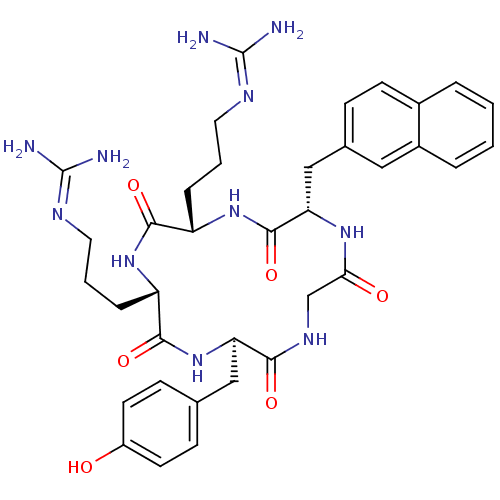
(CHEMBL219474 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-Gly-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C36H47N11O6/c37-35(38)41-15-3-7-26-32(51)45-27(8-4-16-42-36(39)40)33(52)47-28(18-21-10-13-25(48)14-11-21)31(50)43-20-30(49)44-29(34(53)46-26)19-22-9-12-23-5-1-2-6-24(23)17-22/h1-2,5-6,9-14,17,26-29,48H,3-4,7-8,15-16,18-20H2,(H,43,50)(H,44,49)(H,45,51)(H,46,53)(H,47,52)(H4,37,38,41)(H4,39,40,42)/t26-,27-,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588489
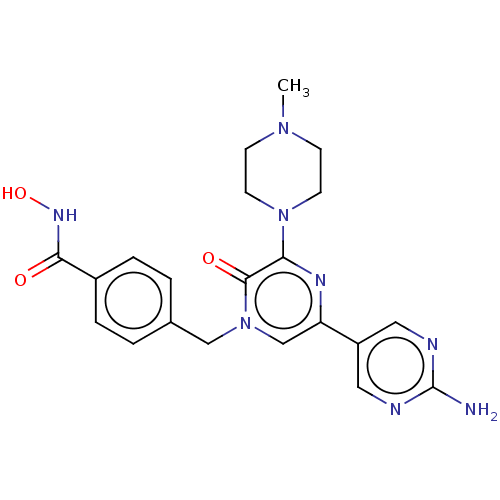
(CHEMBL5201448)Show SMILES CN1CCN(CC1)c1nc(cn(Cc2ccc(cc2)C(=O)NO)c1=O)-c1cnc(N)nc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588488
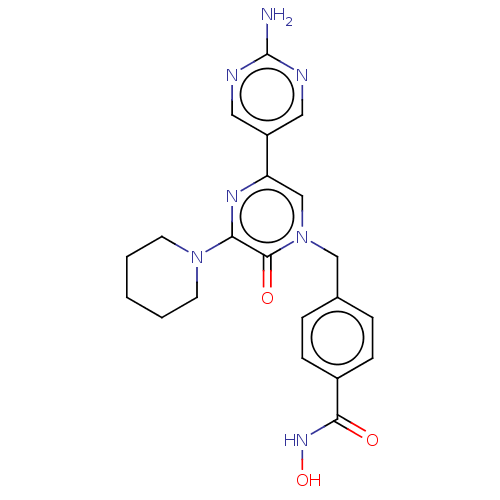
(CHEMBL5192159)Show SMILES Nc1ncc(cn1)-c1cn(Cc2ccc(cc2)C(=O)NO)c(=O)c(n1)N1CCCCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588473
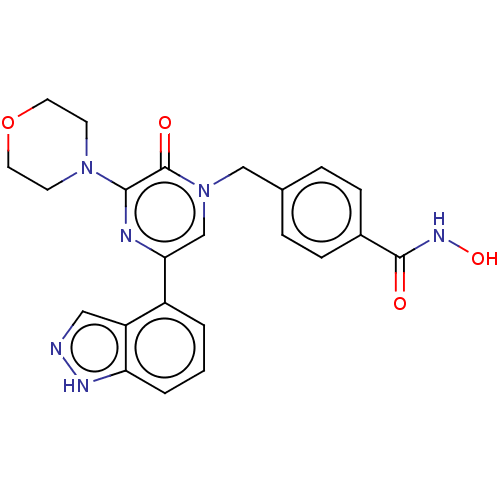
(CHEMBL5180796)Show SMILES ONC(=O)c1ccc(Cn2cc(nc(N3CCOCC3)c2=O)-c2cccc3[nH]ncc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588491
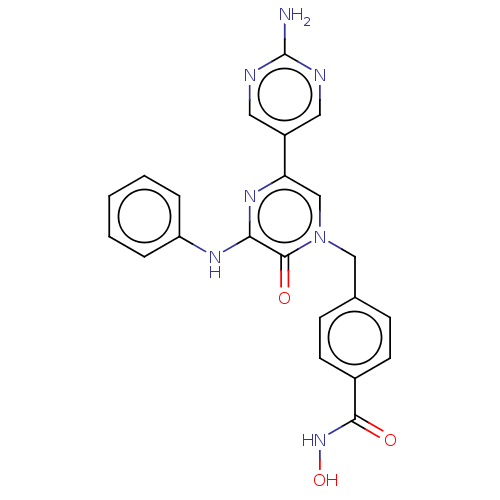
(CHEMBL5184950)Show SMILES Nc1ncc(cn1)-c1cn(Cc2ccc(cc2)C(=O)NO)c(=O)c(Nc2ccccc2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588490
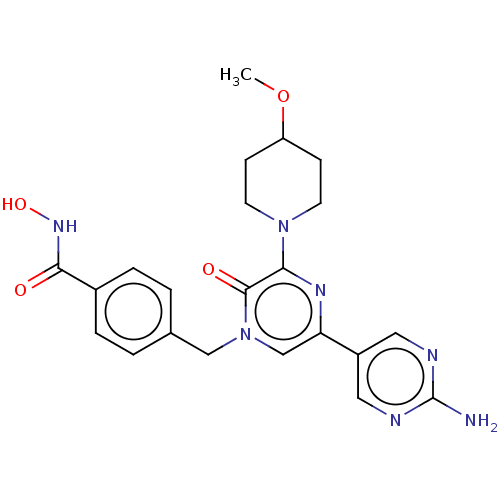
(CHEMBL5190763)Show SMILES COC1CCN(CC1)c1nc(cn(Cc2ccc(cc2)C(=O)NO)c1=O)-c1cnc(N)nc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570277
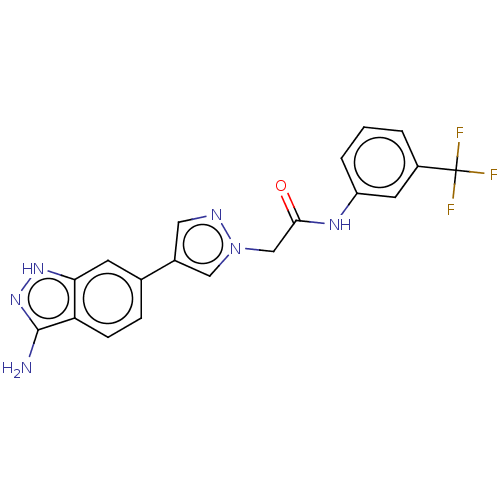
(CHEMBL4861302)Show SMILES Nc1n[nH]c2cc(ccc12)-c1cnn(CC(=O)Nc2cccc(c2)C(F)(F)F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588486

(CHEMBL5180870)Show SMILES ONC(=O)c1ccc(Cn2cc(nc(N3CCOCC3)c2=O)-c2ccoc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50202346

(CHEMBL219339 | cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-Gly-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C36H47N11O6/c37-35(38)41-15-3-7-26-32(51)45-27(8-4-16-42-36(39)40)33(52)47-28(18-21-10-13-25(48)14-11-21)31(50)43-20-30(49)44-29(34(53)46-26)19-22-9-12-23-5-1-2-6-24(23)17-22/h1-2,5-6,9-14,17,26-29,48H,3-4,7-8,15-16,18-20H2,(H,43,50)(H,44,49)(H,45,51)(H,46,53)(H,47,52)(H4,37,38,41)(H4,39,40,42)/t26-,27+,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588471
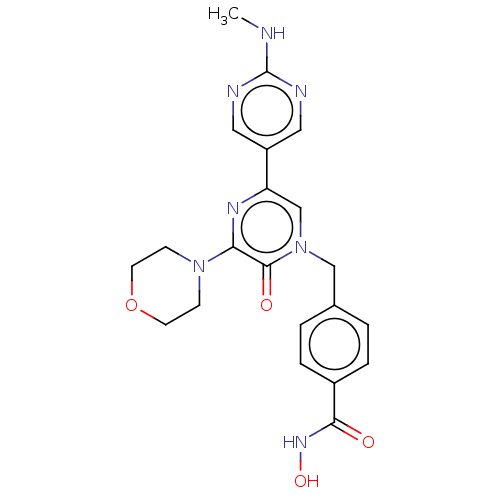
(CHEMBL5202725)Show SMILES CNc1ncc(cn1)-c1cn(Cc2ccc(cc2)C(=O)NO)c(=O)c(n1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50202359
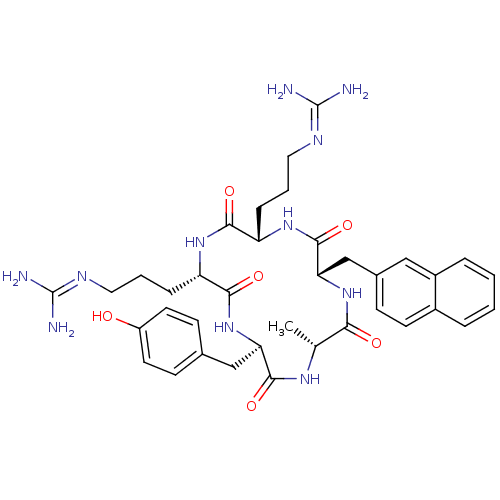
(CHEMBL375990 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-D-Al...)Show SMILES [#6]-[#6@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C37H49N11O6/c1-21-31(50)47-30(20-23-10-13-24-6-2-3-7-25(24)18-23)35(54)46-27(8-4-16-42-36(38)39)32(51)45-28(9-5-17-43-37(40)41)33(52)48-29(34(53)44-21)19-22-11-14-26(49)15-12-22/h2-3,6-7,10-15,18,21,27-30,49H,4-5,8-9,16-17,19-20H2,1H3,(H,44,53)(H,45,51)(H,46,54)(H,47,50)(H,48,52)(H4,38,39,42)(H4,40,41,43)/t21-,27+,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50202345

(CHEMBL373636 | cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-D-Al...)Show SMILES [#6]-[#6@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C37H49N11O6/c1-21-31(50)47-30(20-23-10-13-24-6-2-3-7-25(24)18-23)35(54)46-27(8-4-16-42-36(38)39)32(51)45-28(9-5-17-43-37(40)41)33(52)48-29(34(53)44-21)19-22-11-14-26(49)15-12-22/h2-3,6-7,10-15,18,21,27-30,49H,4-5,8-9,16-17,19-20H2,1H3,(H,44,53)(H,45,51)(H,46,54)(H,47,50)(H,48,52)(H4,38,39,42)(H4,40,41,43)/t21-,27+,28-,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570286
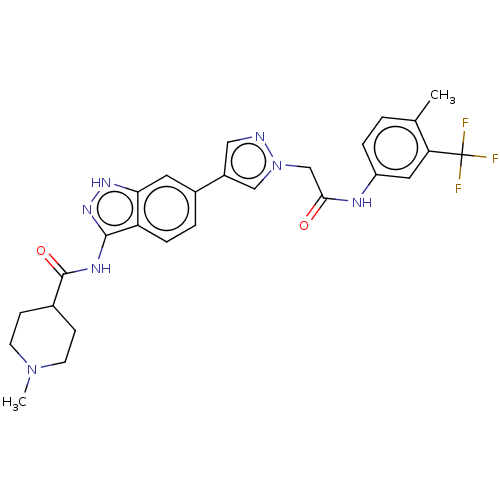
(CHEMBL4874982)Show SMILES CN1CCC(CC1)C(=O)Nc1n[nH]c2cc(ccc12)-c1cnn(CC(=O)Nc2ccc(C)c(c2)C(F)(F)F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570276

(CHEMBL4879197)Show SMILES Nc1n[nH]c2cc(ccc12)-c1cnn(CC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588477
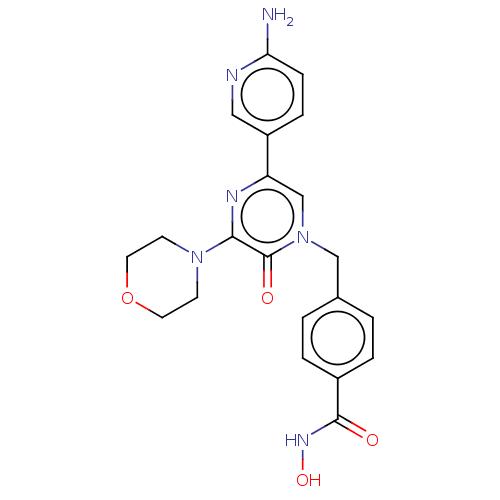
(CHEMBL5191006)Show SMILES Nc1ccc(cn1)-c1cn(Cc2ccc(cc2)C(=O)NO)c(=O)c(n1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570293

(CHEMBL4871126)Show SMILES Cc1ccc(NC(=O)Cc2ccc(s2)-c2ccc3c(NC(=O)C4CC4)n[nH]c3c2)cc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570282
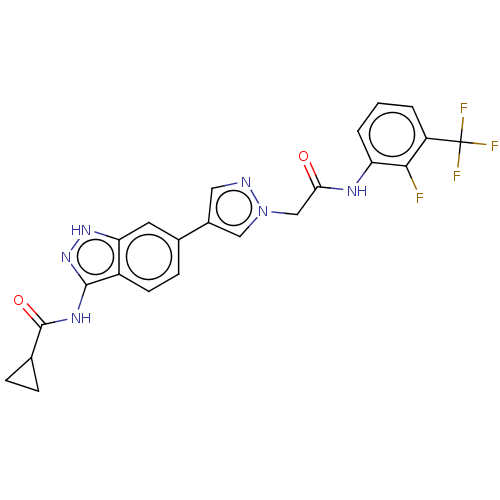
(CHEMBL4860732)Show SMILES Fc1c(NC(=O)Cn2cc(cn2)-c2ccc3c(NC(=O)C4CC4)n[nH]c3c2)cccc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588478
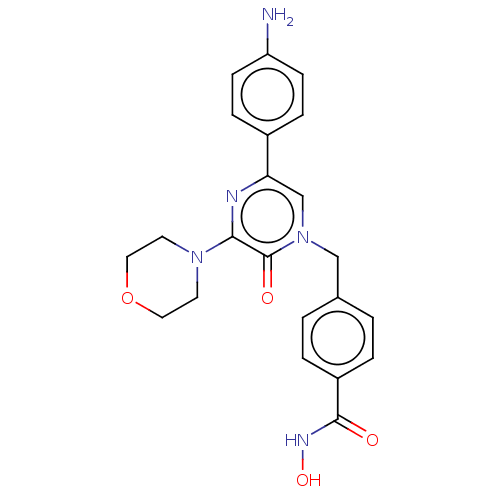
(CHEMBL5174437)Show SMILES Nc1ccc(cc1)-c1cn(Cc2ccc(cc2)C(=O)NO)c(=O)c(n1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380363
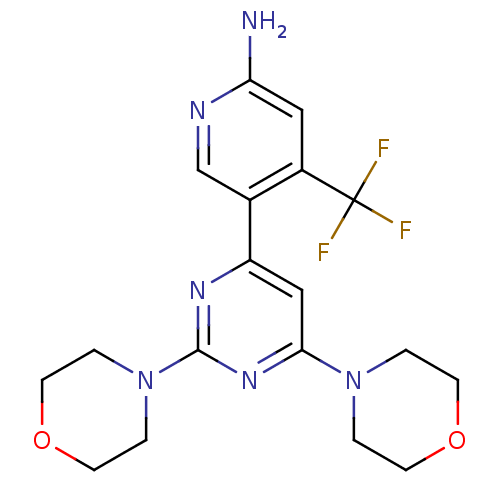
(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588474
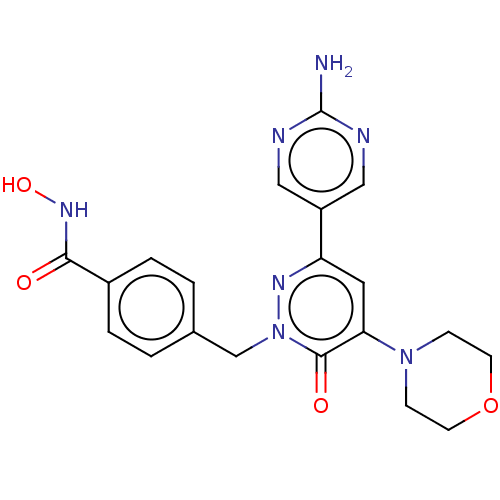
(CHEMBL5192044)Show SMILES Nc1ncc(cn1)-c1cc(N2CCOCC2)c(=O)n(Cc2ccc(cc2)C(=O)NO)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588483
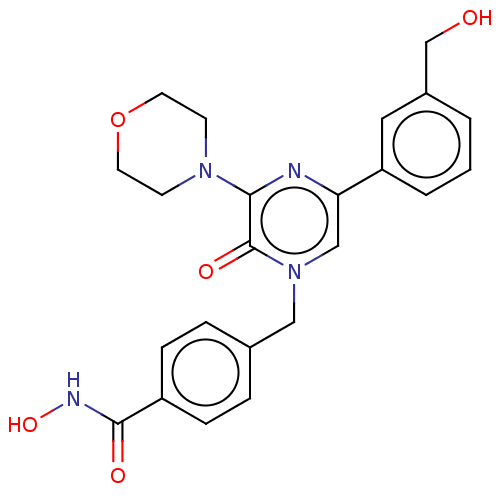
(CHEMBL5206969)Show SMILES OCc1cccc(c1)-c1cn(Cc2ccc(cc2)C(=O)NO)c(=O)c(n1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50588481
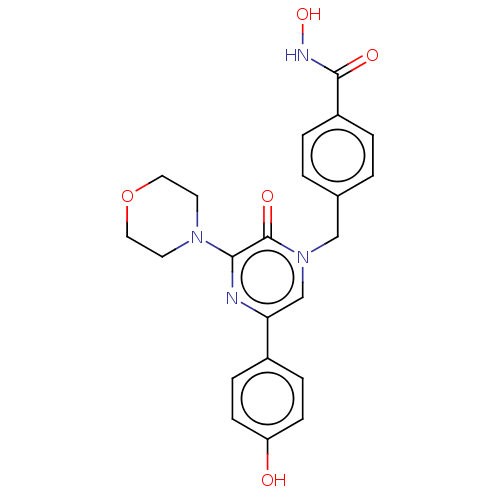
(CHEMBL5175778)Show SMILES ONC(=O)c1ccc(Cn2cc(nc(N3CCOCC3)c2=O)-c2ccc(O)cc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117067
BindingDB Entry DOI: 10.7270/Q2W3818R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50570280

(CHEMBL4846198)Show SMILES FC(F)(F)c1cc(NC(=O)Cn2cc(cn2)-c2ccc3c(NC(=O)C4CC4)n[nH]c3c2)ccc1Cl | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 10 mins followed by kinase substrate addition and measured after 30 mins by caliper mobility shif... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113192
BindingDB Entry DOI: 10.7270/Q2WQ07J0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data