

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50461446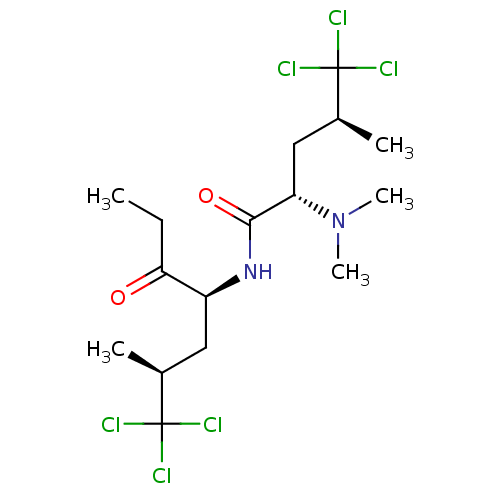 (CHEMBL4227742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate and measured 30 mins post substra... | Bioorg Med Chem Lett 25: 3900-2 (2015) Article DOI: 10.1016/j.bmcl.2015.07.039 BindingDB Entry DOI: 10.7270/Q2KW5HV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by microplate ... | Bioorg Med Chem Lett 26: 315-7 (2016) Article DOI: 10.1016/j.bmcl.2015.12.022 BindingDB Entry DOI: 10.7270/Q2SF2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50461446 (CHEMBL4227742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50232636 ((7E,12E,20Z,18S)-Variabilin | 7E,12E,20Z-Variabili...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50461446 (CHEMBL4227742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human CD45 cytoplasmic domain (584 to 1281 residues) expressed in yeast using pNPP as substrate preincubated for 10 mins followed by su... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50461447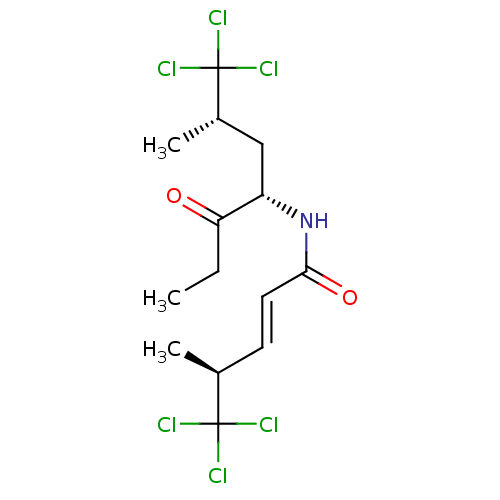 (CHEMBL4228016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using pNPP as substrate assessed as rate of hydrolysis incubated for 10 mins prior to substrate addition measur... | J Nat Prod 78: 1428-33 (2015) Article DOI: 10.1021/acs.jnatprod.5b00375 BindingDB Entry DOI: 10.7270/Q2VH5QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Bioorg Med Chem Lett 25: 904-7 (2015) Article DOI: 10.1016/j.bmcl.2014.12.058 BindingDB Entry DOI: 10.7270/Q29W0H5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50360305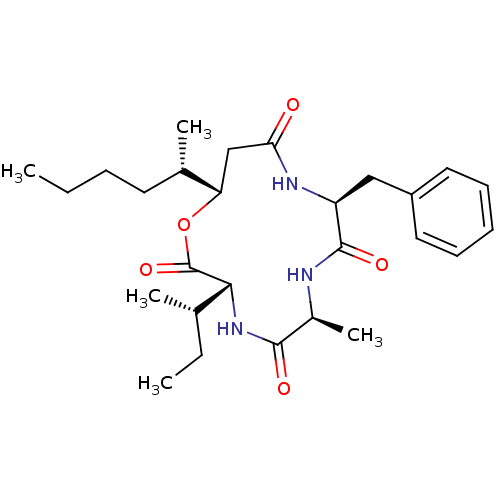 (CHEMBL409855 | US9149492, Beauveriolide III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50461447 (CHEMBL4228016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50232636 ((7E,12E,20Z,18S)-Variabilin | 7E,12E,20Z-Variabili...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human CD45 cytoplasmic domain (584 to 1281 residues) expressed in yeast using pNPP as substrate preincubated for 10 mins followed by su... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50232636 ((7E,12E,20Z,18S)-Variabilin | 7E,12E,20Z-Variabili...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50135267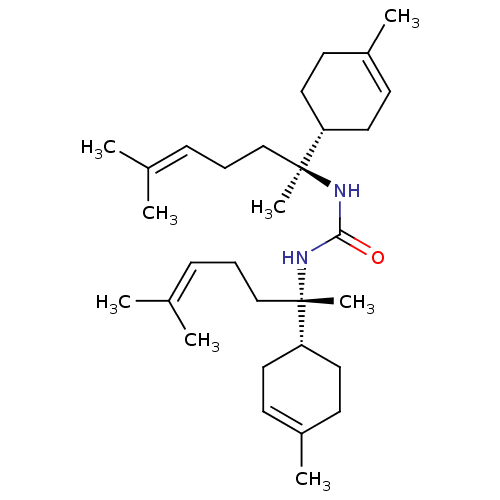 (CHEMBL3746974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by microplate ... | Bioorg Med Chem Lett 26: 315-7 (2016) Article DOI: 10.1016/j.bmcl.2015.12.022 BindingDB Entry DOI: 10.7270/Q2SF2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50461448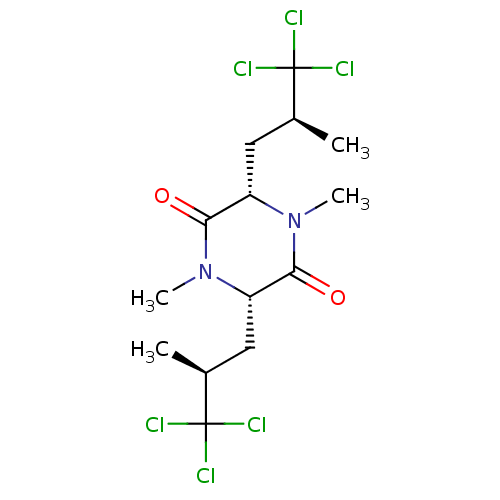 (CHEMBL4227559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50461447 (CHEMBL4228016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50461446 (CHEMBL4227742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50360305 (CHEMBL409855 | US9149492, Beauveriolide III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50360305 (CHEMBL409855 | US9149492, Beauveriolide III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50461447 (CHEMBL4228016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50316020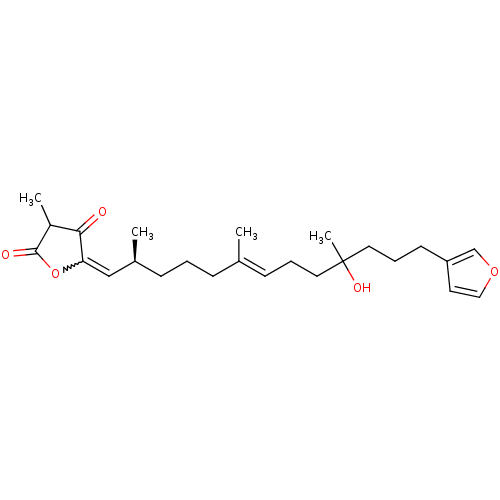 ((12E,20Z,18S)-8-hydroxyvariabilin | 5-((2S)-13-(fu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50461448 (CHEMBL4227559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50461448 (CHEMBL4227559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrs | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50461448 (CHEMBL4227559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min... | Bioorg Med Chem Lett 28: 1911-1914 (2018) Article DOI: 10.1016/j.bmcl.2018.03.077 BindingDB Entry DOI: 10.7270/Q25Q4ZRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50232636 ((7E,12E,20Z,18S)-Variabilin | 7E,12E,20Z-Variabili...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196073 (CHEMBL3919081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50316020 ((12E,20Z,18S)-8-hydroxyvariabilin | 5-((2S)-13-(fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50061005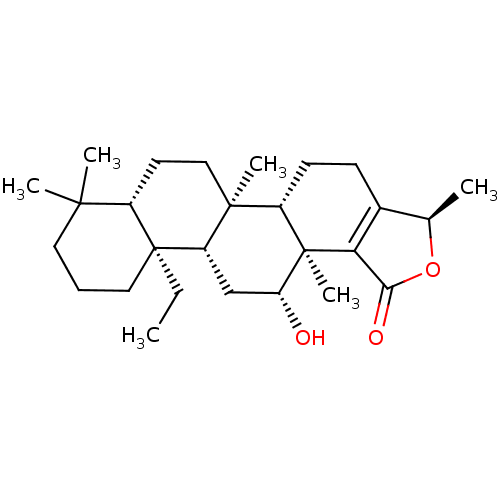 (CHEMBL3393517) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Bioorg Med Chem Lett 25: 904-7 (2015) Article DOI: 10.1016/j.bmcl.2014.12.058 BindingDB Entry DOI: 10.7270/Q29W0H5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50061004 (CHEMBL3393519) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Bioorg Med Chem Lett 25: 904-7 (2015) Article DOI: 10.1016/j.bmcl.2014.12.058 BindingDB Entry DOI: 10.7270/Q29W0H5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50232635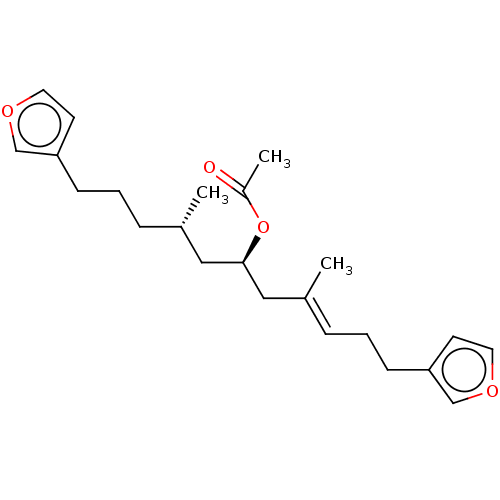 (CHEMBL4089985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115386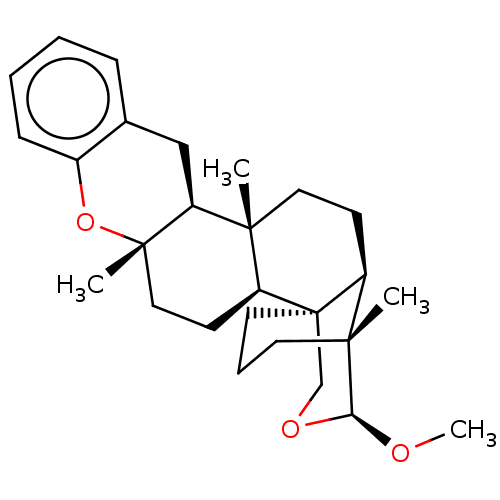 (CHEMBL3608572) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate and measured 30 mins post substra... | Bioorg Med Chem Lett 25: 3900-2 (2015) Article DOI: 10.1016/j.bmcl.2015.07.039 BindingDB Entry DOI: 10.7270/Q2KW5HV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196066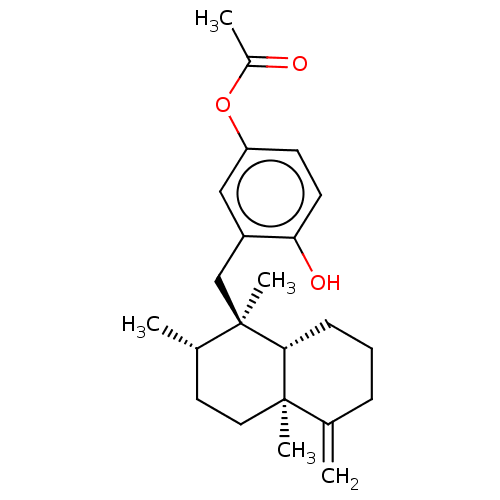 (20-O-Acetylneoavarol | CHEMBL516014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115392 (CHEMBL3608571) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate and measured 30 mins post substra... | Bioorg Med Chem Lett 25: 3900-2 (2015) Article DOI: 10.1016/j.bmcl.2015.07.039 BindingDB Entry DOI: 10.7270/Q2KW5HV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196071 (CHEMBL3971802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115388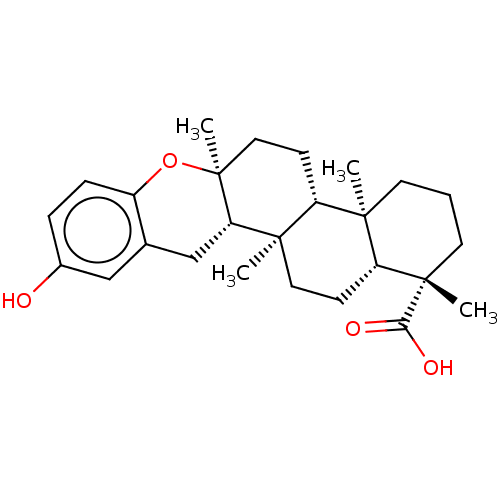 (Strongylophorin-3 | strongylophorine-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate and measured 30 mins post substra... | Bioorg Med Chem Lett 25: 3900-2 (2015) Article DOI: 10.1016/j.bmcl.2015.07.039 BindingDB Entry DOI: 10.7270/Q2KW5HV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50316020 ((12E,20Z,18S)-8-hydroxyvariabilin | 5-((2S)-13-(fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human CD45 cytoplasmic domain (584 to 1281 residues) expressed in yeast using pNPP as substrate preincubated for 10 mins followed by su... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50232635 (CHEMBL4089985) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50316020 ((12E,20Z,18S)-8-hydroxyvariabilin | 5-((2S)-13-(fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196070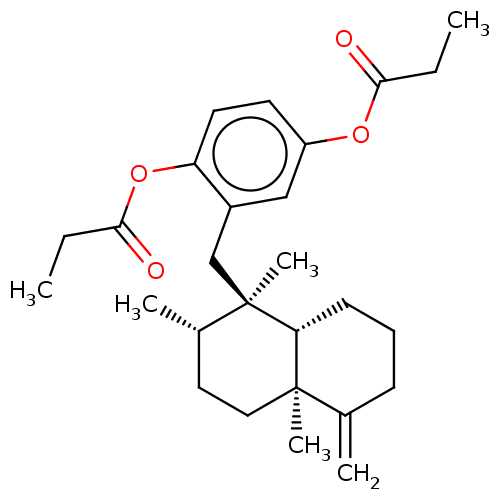 (CHEMBL3962935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196064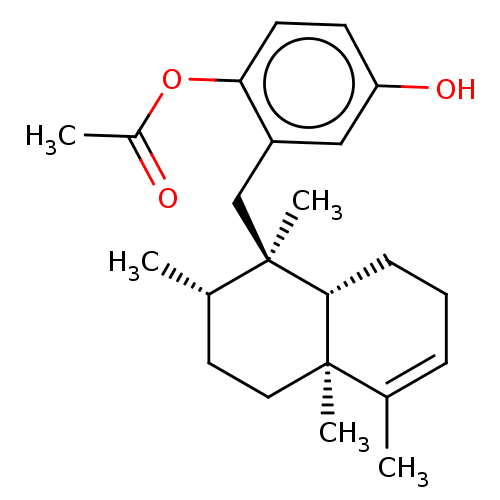 (CHEMBL521017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50232634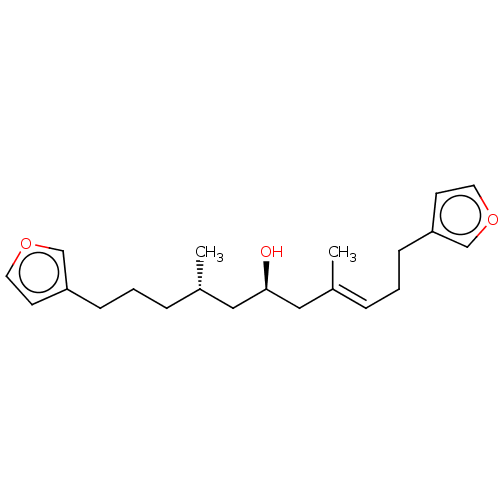 (CHEBI:67726 | Furospongin 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50232634 (CHEBI:67726 | Furospongin 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196067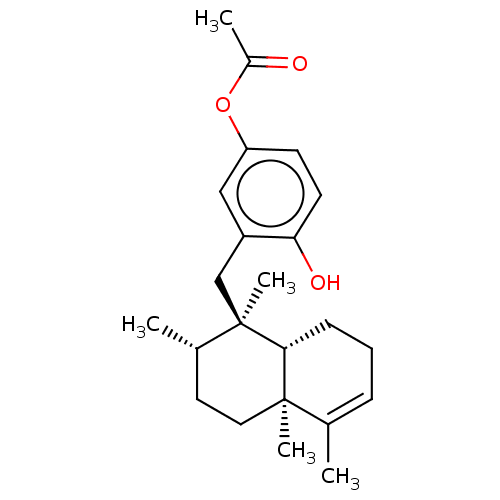 (CHEMBL3893783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196068 (CHEMBL3892140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50232634 (CHEBI:67726 | Furospongin 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 27: 1159-1161 (2017) Article DOI: 10.1016/j.bmcl.2017.01.071 BindingDB Entry DOI: 10.7270/Q2ST7S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115390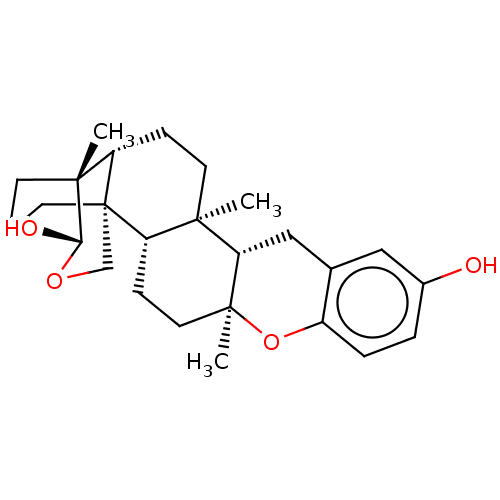 (CHEMBL3608573) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate and measured 30 mins post substra... | Bioorg Med Chem Lett 25: 3900-2 (2015) Article DOI: 10.1016/j.bmcl.2015.07.039 BindingDB Entry DOI: 10.7270/Q2KW5HV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |