

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50277188 (CHEMBL473270 | N1-(biphenyl-3-yl)-N8-hydroxyoctane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal histidine-tagged HDAC6 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50277188 (CHEMBL473270 | N1-(biphenyl-3-yl)-N8-hydroxyoctane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged HDAC1 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123975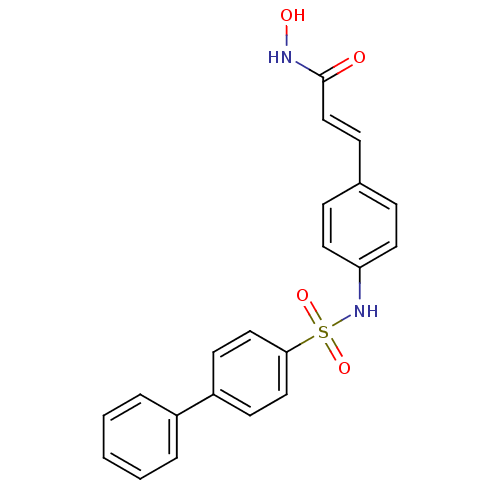 (3-[4-(biphenyl-4-sulfonylamino)-phenyl]-N-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128069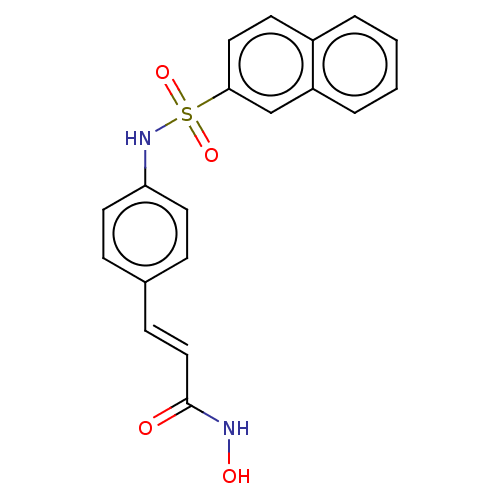 (US8796330, 94) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128072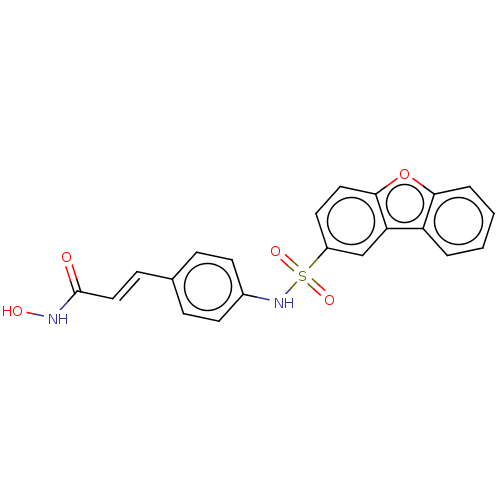 (US8796330, 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123967 (3-[4-(Biphenyl-4-ylsulfamoyl)-phenyl]-N-hydroxy-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123970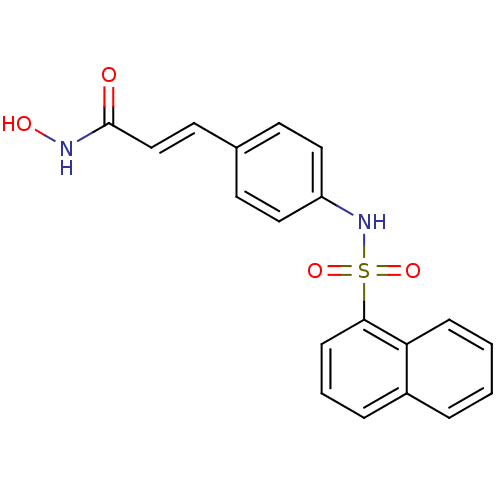 (CHEMBL168961 | N-Hydroxy-3-[4-(naphthalene-1-sulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123965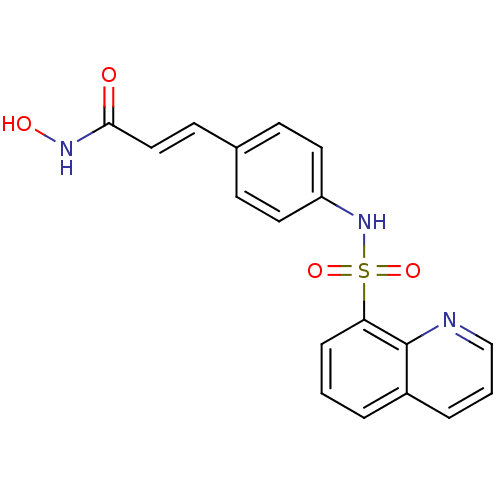 (CHEMBL353839 | N-Hydroxy-3-[4-(quinoline-8-sulfony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128073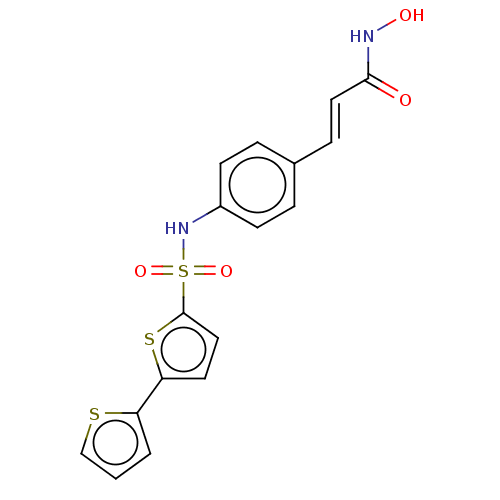 (US8796330, 103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123974 (CHEMBL149534 | N-Hydroxy-3-(4-p-tolylsulfamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123958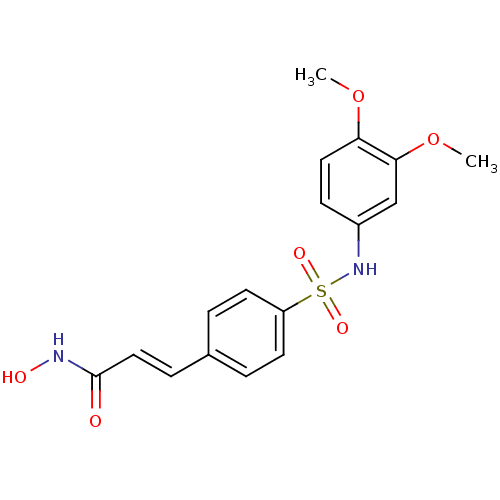 (3-[4-(3,4-Dimethoxy-phenylsulfamoyl)-phenyl]-N-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50277329 ((S)-benzyl 1-(6-methoxyquinolin-8-ylamino)-1-oxo-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal histidine-tagged HDAC6 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50257130 ((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged HDAC1 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105675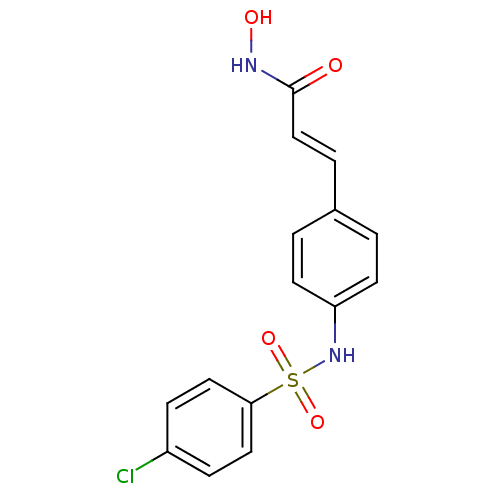 ((E)-3-[4-(4-Chloro-benzenesulfonylamino)-phenyl]-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105689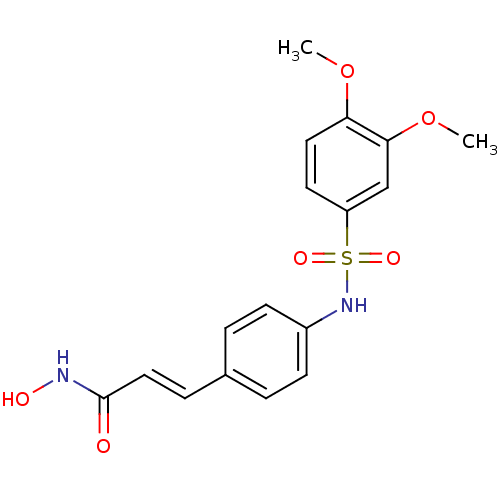 ((E)-3-[4-(3,4-Dimethoxy-benzenesulfonylamino)-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128075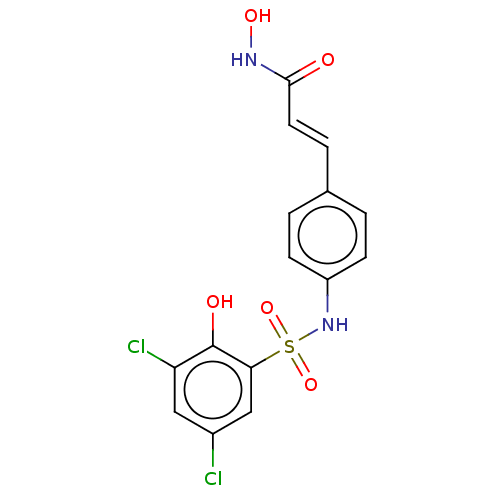 (US8796330, 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50257173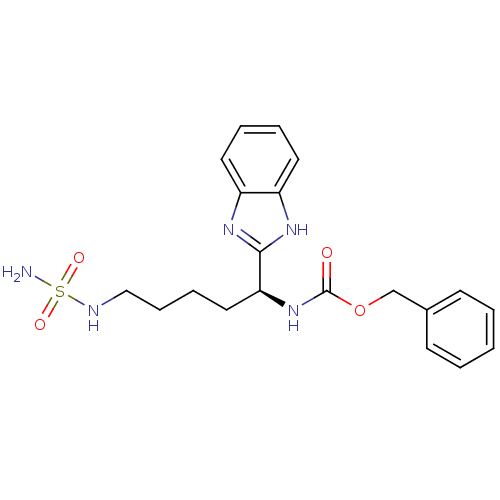 ((S)-benzyl 1-(1H-benzo[d]imidazol-2-yl)-5-(sulfamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged HDAC1 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105688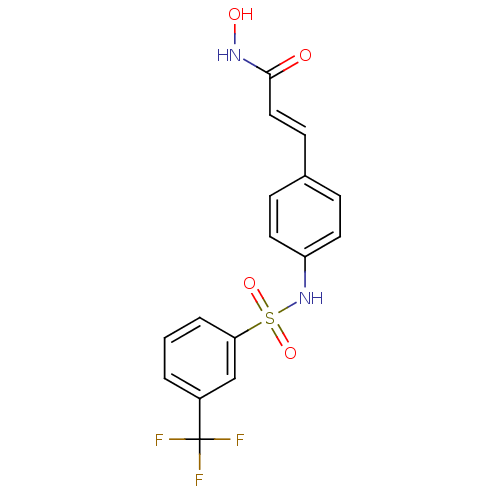 ((E)-N-Hydroxy-3-[4-(3-trifluoromethyl-benzenesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105684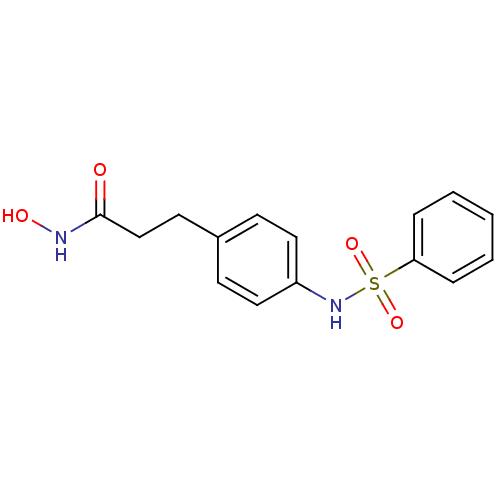 (3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-propio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105686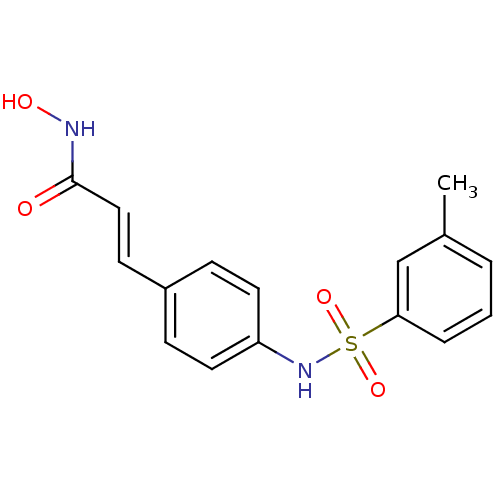 ((E)-N-Hydroxy-3-[4-(toluene-3-sulfonylamino)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128084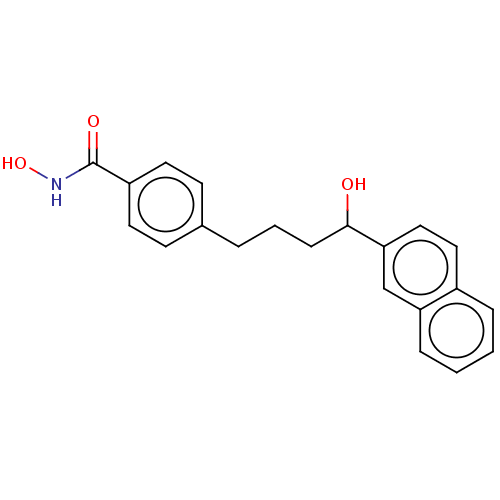 (US8796330, 149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50256996 ((S)-benzyl 1-(4-(4-methoxyphenyl)thiazol-2-ylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged HDAC1 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105696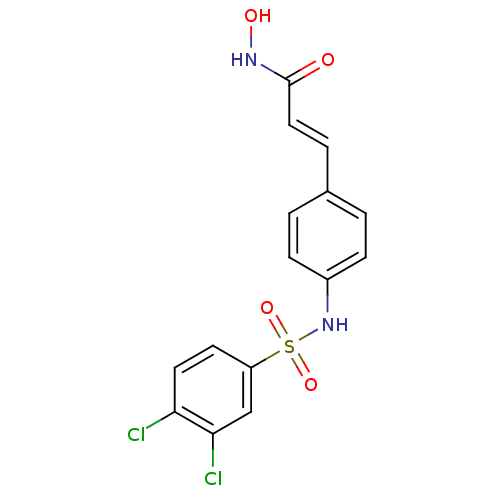 ((E)-3-[4-(3,4-Dichloro-benzenesulfonylamino)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50257091 ((S)-2-(2-phenylacetamido)-N-(4-phenylthiazol-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50257046 ((S)-benzyl 1-(4-(4-chlorophenyl)thiazol-2-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged HDAC1 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50256888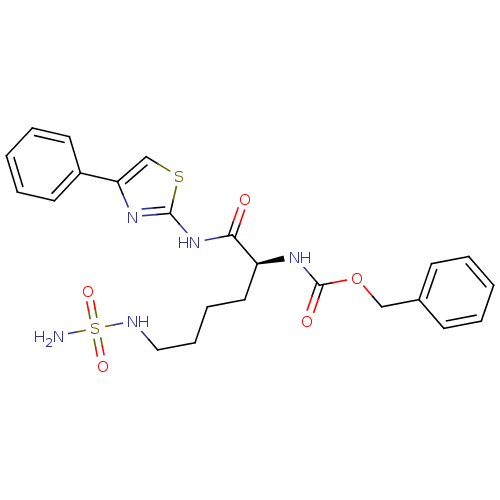 ((S)-benzyl 1-oxo-1-(4-phenylthiazol-2-ylamino)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal FLAG-tagged HDAC1 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50256888 ((S)-benzyl 1-oxo-1-(4-phenylthiazol-2-ylamino)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged HDAC1 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50256888 ((S)-benzyl 1-oxo-1-(4-phenylthiazol-2-ylamino)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50256888 ((S)-benzyl 1-oxo-1-(4-phenylthiazol-2-ylamino)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal histidine-tagged HDAC6 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50256945 ((S)-benzyl 1-(benzo[d]thiazol-2-ylamino)-1-oxo-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50256887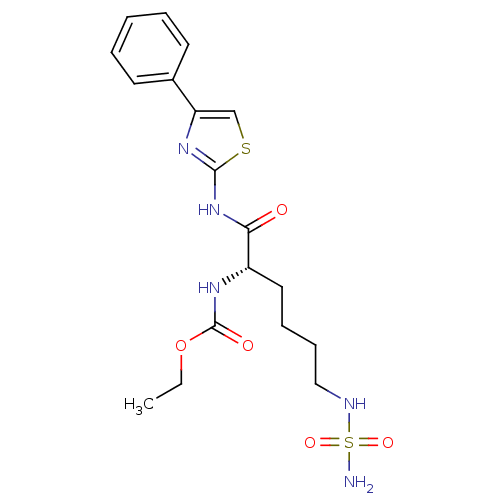 ((S)-ethyl 1-oxo-1-(4-phenylthiazol-2-ylamino)-6-(s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105692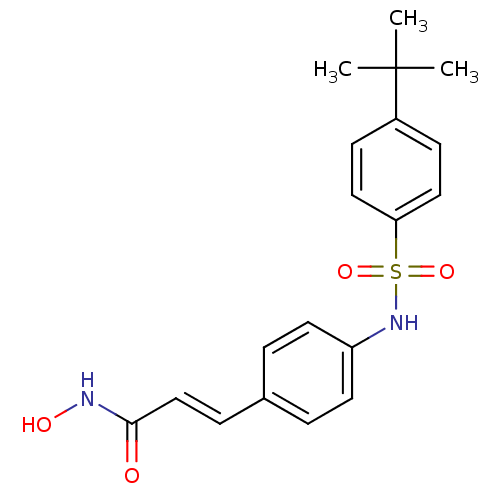 ((E)-3-[4-(4-tert-Butyl-benzenesulfonylamino)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105691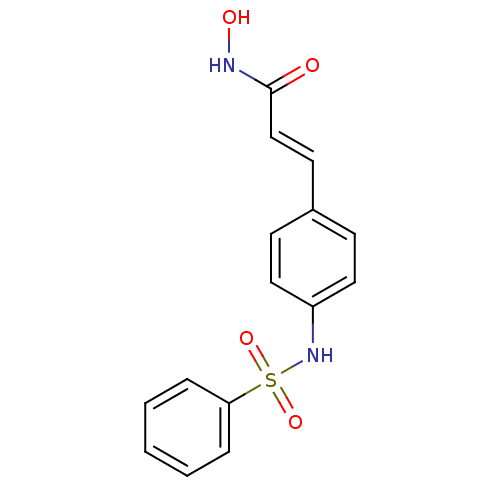 ((E)-3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105678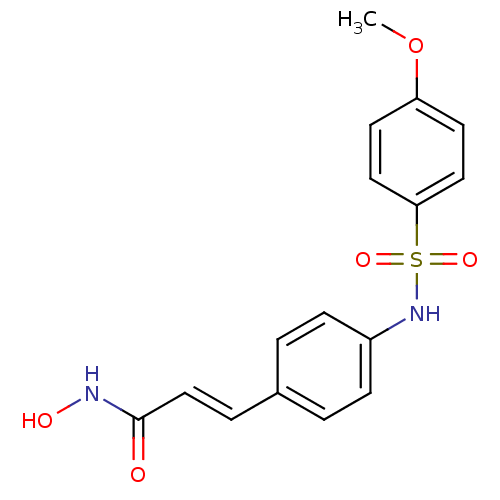 ((E)-N-Hydroxy-3-[4-(4-methoxy-benzenesulfonylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal histidine-tagged HDAC6 expressed in baculovirus by fluorimetry | Bioorg Med Chem Lett 19: 336-40 (2008) Article DOI: 10.1016/j.bmcl.2008.11.081 BindingDB Entry DOI: 10.7270/Q2416WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128099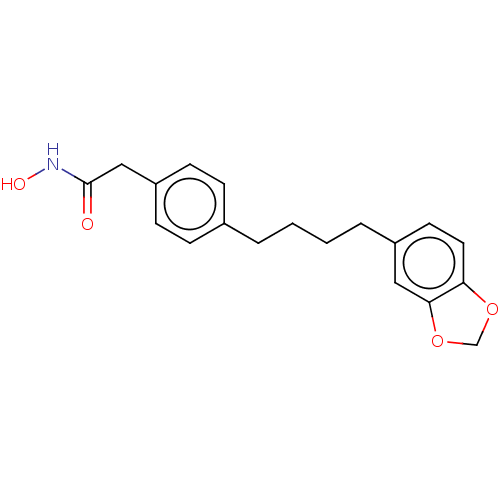 (US8796330, 187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50256996 ((S)-benzyl 1-(4-(4-methoxyphenyl)thiazol-2-ylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50257223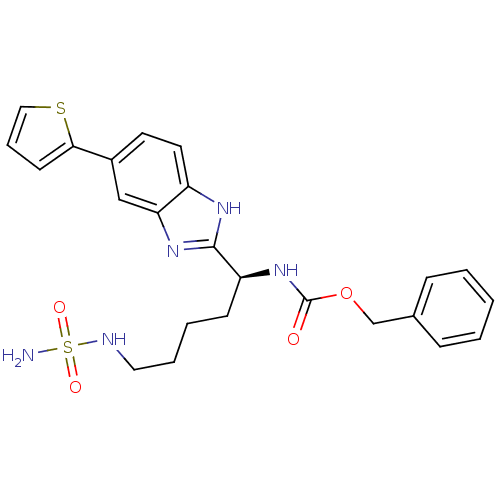 ((S)-benzyl 5-(sulfamoylamino)-1-(5-(thiophen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged HDAC1 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128070 (US8796330, 95) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM128099 (US8796330, 187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50257046 ((S)-benzyl 1-(4-(4-chlorophenyl)thiazol-2-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50256947 ((S)-3-chlorobenzyl 1-oxo-1-(4-phenylthiazol-2-ylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123977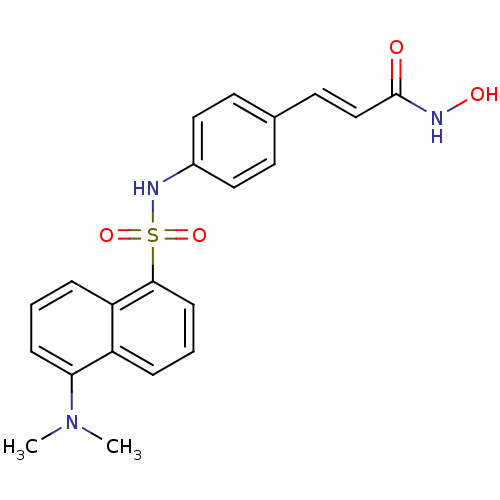 (3-[4-(5-Dimethylamino-naphthalene-1-sulfonylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50257224 ((S)-benzyl 1-(2-phenyl-1H-imidazol-4-yl)-5-(sulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged HDAC1 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105677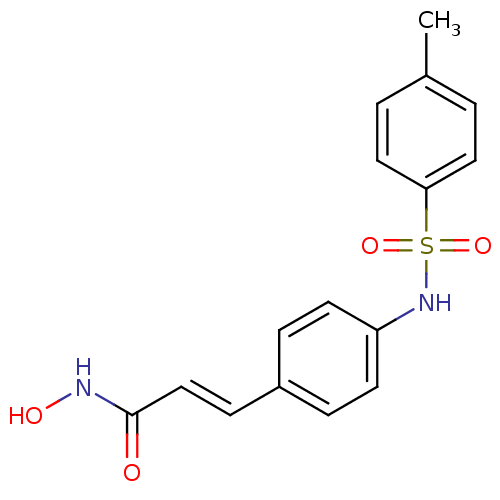 ((E)-N-Hydroxy-3-[4-(toluene-4-sulfonylamino)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128065 (US8796330, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105681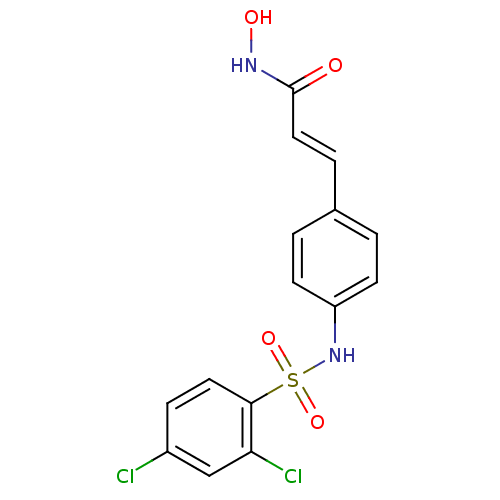 ((E)-3-[4-(2,4-Dichloro-benzenesulfonylamino)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM128077 (US8796330, 128) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50257225 ((S)-benzyl 1-(5-phenyl-1H-1,2,4-triazol-3-yl)-5-(s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged HDAC6 expressed in baculovirus | Bioorg Med Chem Lett 19: 1866-70 (2009) Article DOI: 10.1016/j.bmcl.2009.02.075 BindingDB Entry DOI: 10.7270/Q2PV6K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 188 total ) | Next | Last >> |