

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123975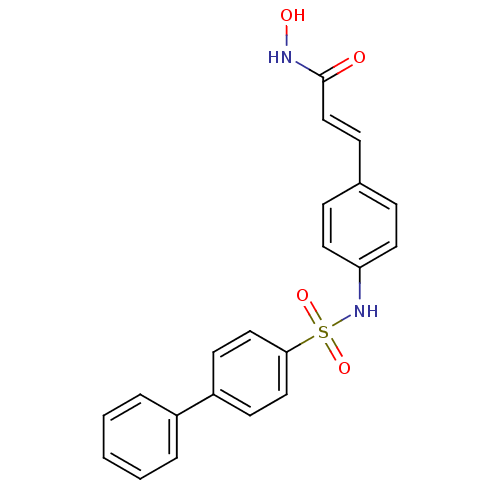 (3-[4-(biphenyl-4-sulfonylamino)-phenyl]-N-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123975 (3-[4-(biphenyl-4-sulfonylamino)-phenyl]-N-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc Curated by ChEMBL | Assay Description Inhibitory concentration against human Histone deacetylase 1 | J Med Chem 46: 5097-116 (2003) Article DOI: 10.1021/jm0303094 BindingDB Entry DOI: 10.7270/Q2MP5413 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123975 (3-[4-(biphenyl-4-sulfonylamino)-phenyl]-N-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||