 Found 93 hits with Last Name = 'al-sharmma' and Initial = 'h'
Found 93 hits with Last Name = 'al-sharmma' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50106206

(CHEMBL3598099)Show SMILES C[C@@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,wD:12.12,1.0,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50106206

(CHEMBL3598099)Show SMILES C[C@@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,wD:12.12,1.0,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Astemizole from human ERG |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106150

(CHEMBL3598081)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H35NO6S/c1-23(2,3)30-22(25)24-15-13-20(14-16-24)29-18-7-5-17(6-8-18)28-19-9-11-21(12-10-19)31(4,26)27/h9-12,17-18,20H,5-8,13-16H2,1-4H3/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106151
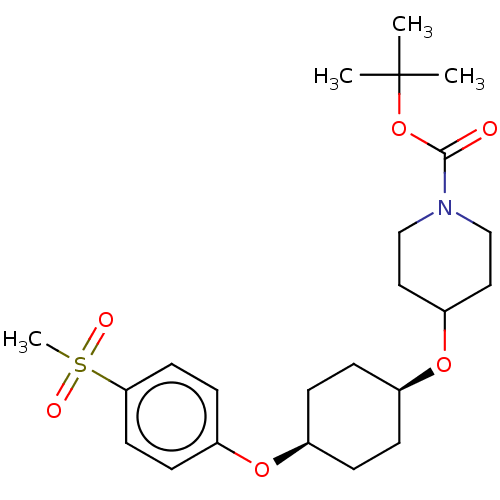
(CHEMBL3598082)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@H](CC1)Oc1ccc(cc1)S(C)(=O)=O |r,wU:14.14,17.21,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H35NO6S/c1-23(2,3)30-22(25)24-15-13-20(14-16-24)29-18-7-5-17(6-8-18)28-19-9-11-21(12-10-19)31(4,26)27/h9-12,17-18,20H,5-8,13-16H2,1-4H3/t17-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 266 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106152

(CHEMBL3598083)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cc1F)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;5.34,-2.76,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H34FNO6S/c1-23(2,3)31-22(26)25-13-11-18(12-14-25)29-16-5-7-17(8-6-16)30-21-10-9-19(15-20(21)24)32(4,27)28/h9-10,15-18H,5-8,11-14H2,1-4H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106153
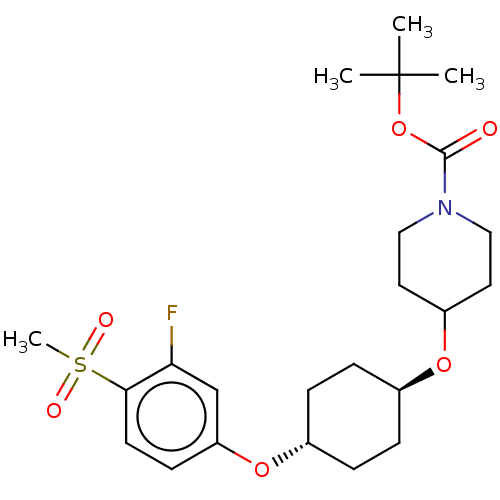
(CHEMBL3598084)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(c(F)c1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;7.74,-1.37,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H34FNO6S/c1-23(2,3)31-22(26)25-13-11-18(12-14-25)29-16-5-7-17(8-6-16)30-19-9-10-21(20(24)15-19)32(4,27)28/h9-10,15-18H,5-8,11-14H2,1-4H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106154
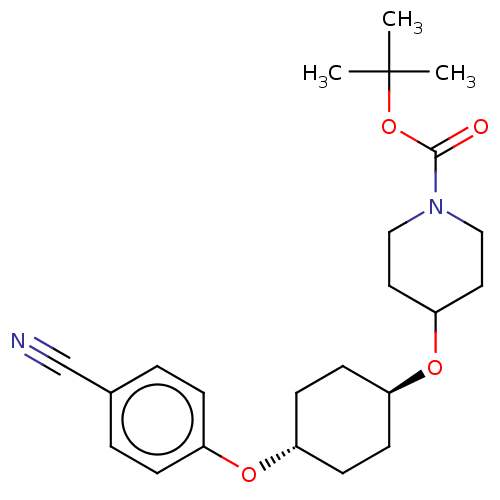
(CHEMBL3598085)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C#N |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,)| Show InChI InChI=1S/C23H32N2O4/c1-23(2,3)29-22(26)25-14-12-21(13-15-25)28-20-10-8-19(9-11-20)27-18-6-4-17(16-24)5-7-18/h4-7,19-21H,8-15H2,1-3H3/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106155
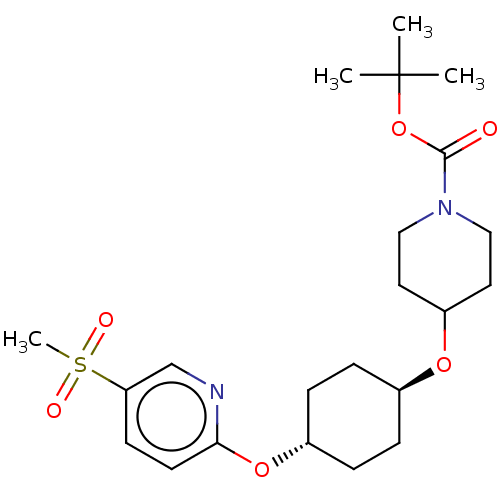
(CHEMBL3598086)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C22H34N2O6S/c1-22(2,3)30-21(25)24-13-11-18(12-14-24)28-16-5-7-17(8-6-16)29-20-10-9-19(15-23-20)31(4,26)27/h9-10,15-18H,5-8,11-14H2,1-4H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106156
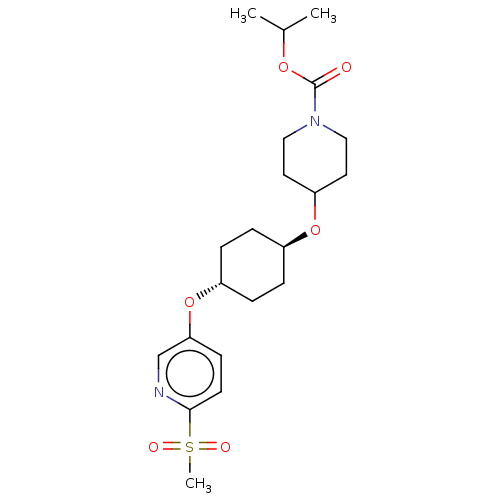
(CHEMBL3598087)Show SMILES CC(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(nc1)S(C)(=O)=O |r,wU:16.20,wD:13.13,(-.23,10.62,;-1.3,10.01,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C21H32N2O6S/c1-15(2)27-21(24)23-12-10-18(11-13-23)28-16-4-6-17(7-5-16)29-19-8-9-20(22-14-19)30(3,25)26/h8-9,14-18H,4-7,10-13H2,1-3H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106157
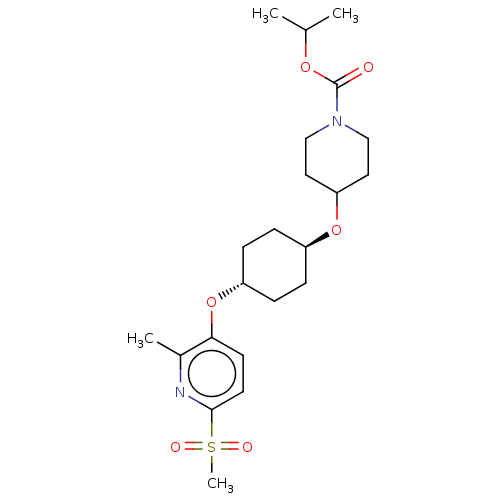
(CHEMBL3598088)Show SMILES CC(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(nc1C)S(C)(=O)=O |r,wU:16.20,wD:13.13,(-.23,10.62,;-1.3,10.01,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;5.34,-2.76,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C22H34N2O6S/c1-15(2)28-22(25)24-13-11-19(12-14-24)29-17-5-7-18(8-6-17)30-20-9-10-21(23-16(20)3)31(4,26)27/h9-10,15,17-19H,5-8,11-14H2,1-4H3/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106158
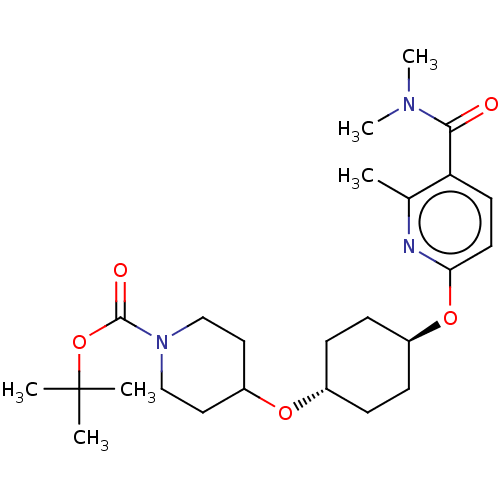
(CHEMBL3598089)Show SMILES CN(C)C(=O)c1ccc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)OC(C)(C)C)nc1C |r,wU:10.9,wD:13.16,(9.05,3.72,;7.99,3.1,;6.92,3.71,;8,1.56,;9.07,.95,;6.67,.78,;5.33,1.55,;4,.77,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;-.23,10.62,;-1.29,11.24,;-2.36,10.63,;5.34,-1.53,;6.67,-.76,;7.74,-1.37,)| Show InChI InChI=1S/C25H39N3O5/c1-17-21(23(29)27(5)6)11-12-22(26-17)32-19-9-7-18(8-10-19)31-20-13-15-28(16-14-20)24(30)33-25(2,3)4/h11-12,18-20H,7-10,13-16H2,1-6H3/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 262 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106159

(CHEMBL3598090)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C21H33N3O6S/c1-21(2,3)30-20(25)24-11-9-17(10-12-24)28-15-5-7-16(8-6-15)29-18-13-23-19(14-22-18)31(4,26)27/h13-17H,5-12H2,1-4H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106160
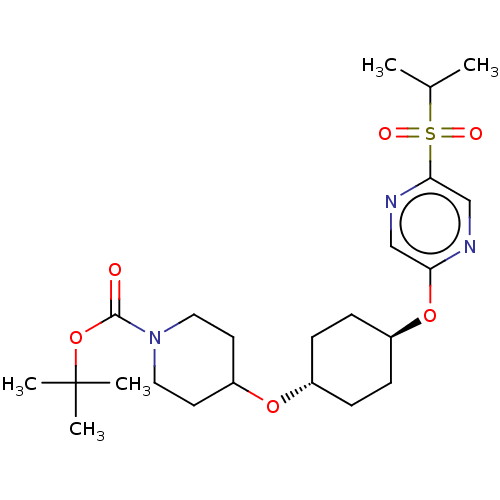
(CHEMBL3598091)Show SMILES CC(C)S(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)OC(C)(C)C)cn1 |r,wU:11.10,wD:14.17,(10.4,1.41,;9.33,.79,;9.34,-.44,;8,1.56,;7.99,2.79,;9.06,2.18,;6.67,.78,;5.33,1.55,;4,.77,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;-.23,10.62,;-1.29,11.24,;-2.36,10.63,;5.34,-1.53,;6.67,-.76,)| Show InChI InChI=1S/C23H37N3O6S/c1-16(2)33(28,29)21-15-24-20(14-25-21)31-18-8-6-17(7-9-18)30-19-10-12-26(13-11-19)22(27)32-23(3,4)5/h14-19H,6-13H2,1-5H3/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106161
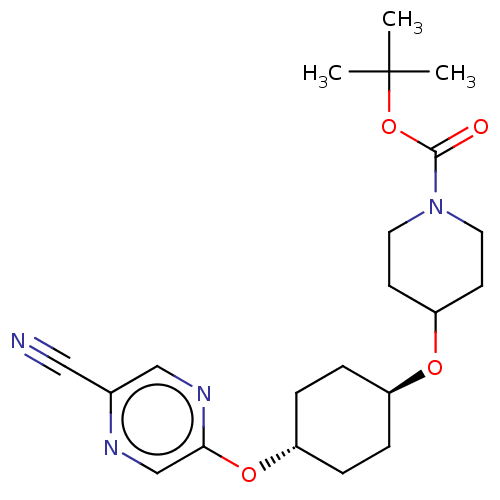
(CHEMBL3598092)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)C#N |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,)| Show InChI InChI=1S/C21H30N4O4/c1-21(2,3)29-20(26)25-10-8-18(9-11-25)27-16-4-6-17(7-5-16)28-19-14-23-15(12-22)13-24-19/h13-14,16-18H,4-11H2,1-3H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106162
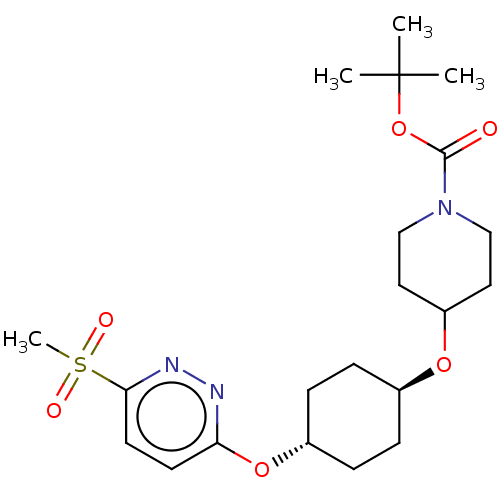
(CHEMBL3598093)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(nn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C21H33N3O6S/c1-21(2,3)30-20(25)24-13-11-17(12-14-24)28-15-5-7-16(8-6-15)29-18-9-10-19(23-22-18)31(4,26)27/h9-10,15-17H,5-8,11-14H2,1-4H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 651 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106163
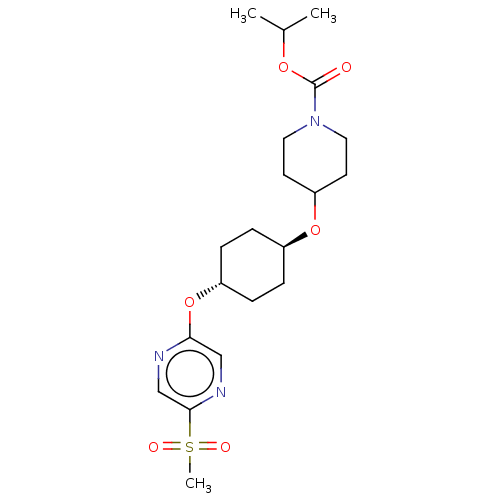
(CHEMBL3598094)Show SMILES CC(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:16.20,wD:13.13,(-.23,10.62,;-1.3,10.01,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C20H31N3O6S/c1-14(2)27-20(24)23-10-8-17(9-11-23)28-15-4-6-16(7-5-15)29-18-12-22-19(13-21-18)30(3,25)26/h12-17H,4-11H2,1-3H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106198

(CHEMBL3598095)Show SMILES CC1(CC1)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.22,wD:14.15,(-.23,10.62,;-1.3,10.01,;-2.72,9.95,;-1.94,11.28,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C21H31N3O6S/c1-21(9-10-21)30-20(25)24-11-7-17(8-12-24)28-15-3-5-16(6-4-15)29-18-13-23-19(14-22-18)31(2,26)27/h13-17H,3-12H2,1-2H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106203
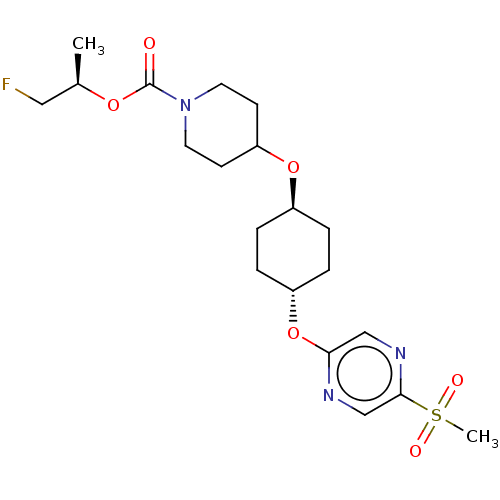
(CHEMBL3598096)Show SMILES C[C@H](CF)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,1.0,(-2.36,10.63,;-1.3,10.01,;.04,10.77,;.05,12.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C20H30FN3O6S/c1-14(11-21)28-20(25)24-9-7-17(8-10-24)29-15-3-5-16(6-4-15)30-18-12-23-19(13-22-18)31(2,26)27/h12-17H,3-11H2,1-2H3/t14-,15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106204

(CHEMBL3598097)Show SMILES C[C@@H](CF)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.21,1.0,wD:14.14,(-2.36,10.63,;-1.3,10.01,;.04,10.77,;.05,12.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C20H30FN3O6S/c1-14(11-21)28-20(25)24-9-7-17(8-10-24)29-15-3-5-16(6-4-15)30-18-12-23-19(13-22-18)31(2,26)27/h12-17H,3-11H2,1-2H3/t14-,15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106205
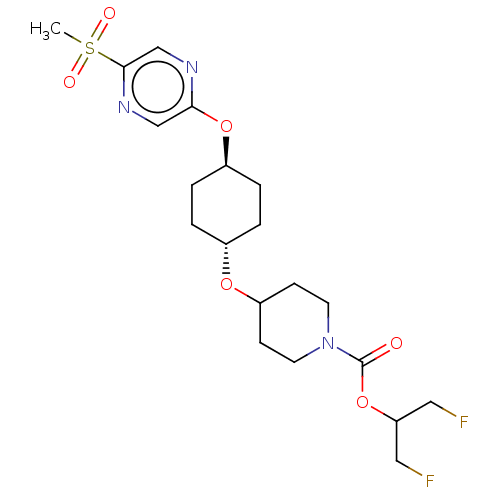
(CHEMBL3598098)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)OC(CF)CF)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;.04,10.77,;.05,12.01,;-2.63,10.79,;-2.62,12.02,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C20H29F2N3O6S/c1-32(27,28)19-13-23-18(12-24-19)30-15-4-2-14(3-5-15)29-16-6-8-25(9-7-16)20(26)31-17(10-21)11-22/h12-17H,2-11H2,1H3/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106206

(CHEMBL3598099)Show SMILES C[C@@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,wD:12.12,1.0,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106207
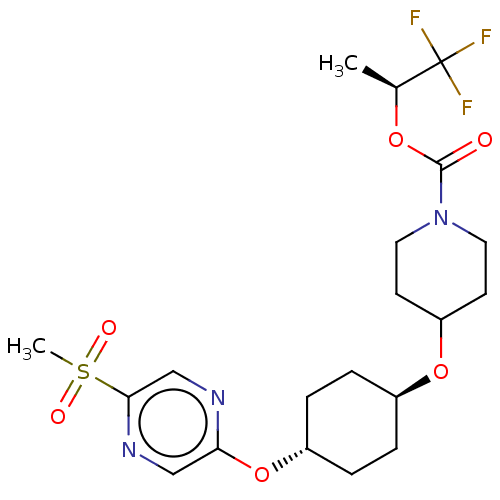
(CHEMBL3598100)Show SMILES C[C@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,1.0,wD:12.12,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106208

(CHEMBL3598101)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,;-2.63,10.78,;-3.7,10.17,;-2.63,12.02,;-3.7,11.4,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C20H25F6N3O6S/c1-36(31,32)16-11-27-15(10-28-16)34-13-4-2-12(3-5-13)33-14-6-8-29(9-7-14)18(30)35-17(19(21,22)23)20(24,25)26/h10-14,17H,2-9H2,1H3/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106209
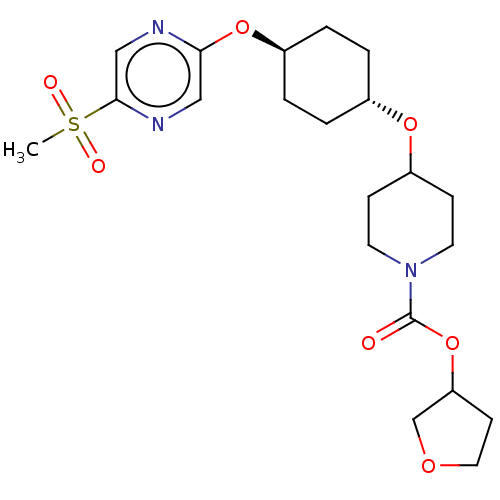
(CHEMBL3598102)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)OC2CCOC2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;-2.54,10.9,;-2.06,12.36,;-.52,12.35,;-.05,10.89,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C21H31N3O7S/c1-32(26,27)20-13-22-19(12-23-20)30-16-4-2-15(3-5-16)29-17-6-9-24(10-7-17)21(25)31-18-8-11-28-14-18/h12-13,15-18H,2-11,14H2,1H3/t15-,16-,18? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 427 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106210
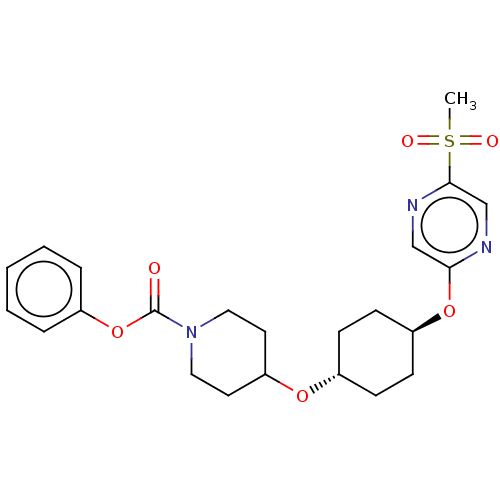
(CHEMBL3598103)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)Oc2ccccc2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;.04,10.78,;.04,12.31,;-1.29,13.09,;-2.62,12.32,;-2.63,10.78,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C23H29N3O6S/c1-33(28,29)22-16-24-21(15-25-22)31-19-9-7-18(8-10-19)30-20-11-13-26(14-12-20)23(27)32-17-5-3-2-4-6-17/h2-6,15-16,18-20H,7-14H2,1H3/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106211
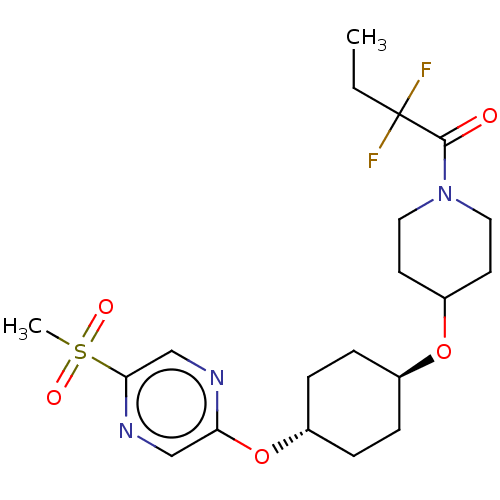
(CHEMBL3598104)Show SMILES CCC(F)(F)C(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.31,8.47,;-.24,9.08,;-.24,7.85,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C20H29F2N3O5S/c1-3-20(21,22)19(26)25-10-8-16(9-11-25)29-14-4-6-15(7-5-14)30-17-12-24-18(13-23-17)31(2,27)28/h12-16H,3-11H2,1-2H3/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106212
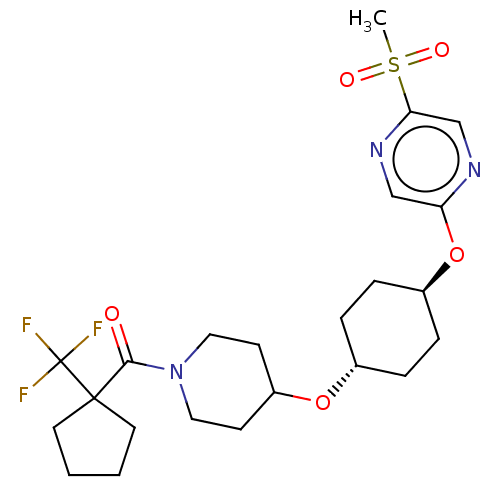
(CHEMBL3598105)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)C2(CCCC2)C(F)(F)F)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;.05,9.12,;1.07,7.97,;.29,6.64,;-1.21,6.97,;-1.3,10.01,;-2.36,10.63,;-1.29,11.24,;-.23,10.62,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C23H32F3N3O5S/c1-35(31,32)20-15-27-19(14-28-20)34-17-6-4-16(5-7-17)33-18-8-12-29(13-9-18)21(30)22(23(24,25)26)10-2-3-11-22/h14-18H,2-13H2,1H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106213

(CHEMBL3598106)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;.11,8.53,;-.66,7.2,;-1.3,10.01,;-2.36,10.63,;-1.29,11.24,;-.23,10.62,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C21H28F3N3O5S/c1-33(29,30)18-13-25-17(12-26-18)32-15-4-2-14(3-5-15)31-16-6-10-27(11-7-16)19(28)20(8-9-20)21(22,23)24/h12-16H,2-11H2,1H3/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106214

(CHEMBL3598107)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC(F)(F)F)CC2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-2.65,7.7,;-1.31,8.47,;-1.3,9.7,;-.24,7.85,;-.24,9.08,;-1.32,5.39,;-1.33,3.85,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C18H26F3N3O4S/c1-29(25,26)17-11-22-16(10-23-17)28-14-4-2-13(3-5-14)27-15-6-8-24(9-7-15)12-18(19,20)21/h10-11,13-15H,2-9,12H2,1H3/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 776 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106215
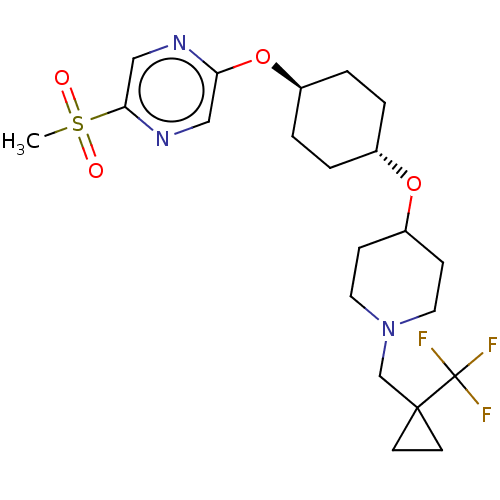
(CHEMBL3598108)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC3(CC3)C(F)(F)F)CC2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-2.65,7.7,;-1.31,8.47,;.11,8.53,;-.66,7.2,;-1.3,10.01,;-2.36,10.63,;-1.29,11.24,;-.23,10.62,;-1.32,5.39,;-1.33,3.85,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C21H30F3N3O4S/c1-32(28,29)19-13-25-18(12-26-19)31-16-4-2-15(3-5-16)30-17-6-10-27(11-7-17)14-20(8-9-20)21(22,23)24/h12-13,15-17H,2-11,14H2,1H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106216
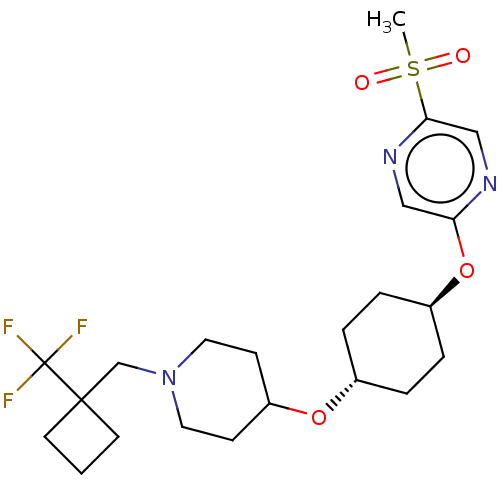
(CHEMBL3598109)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC3(CCC3)C(F)(F)F)CC2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-2.65,7.7,;-1.31,8.47,;.1,8.91,;.49,7.42,;-1,7.03,;-1.3,10.01,;-2.36,10.63,;-1.29,11.24,;-.23,10.62,;-1.32,5.39,;-1.33,3.85,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C22H32F3N3O4S/c1-33(29,30)20-14-26-19(13-27-20)32-17-5-3-16(4-6-17)31-18-7-11-28(12-8-18)15-21(9-2-10-21)22(23,24)25/h13-14,16-18H,2-12,15H2,1H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106217
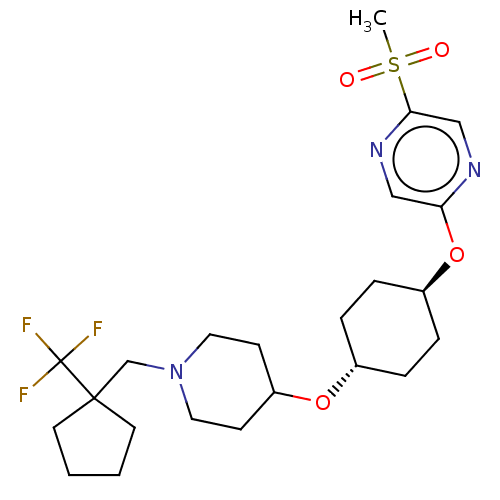
(CHEMBL3598110)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC3(CCCC3)C(F)(F)F)CC2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-2.65,7.7,;-1.31,8.47,;.05,9.12,;1.07,7.97,;.29,6.64,;-1.21,6.97,;-1.3,10.01,;-2.36,10.63,;-1.29,11.24,;-.23,10.62,;-1.32,5.39,;-1.33,3.85,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C23H34F3N3O4S/c1-34(30,31)21-15-27-20(14-28-21)33-18-6-4-17(5-7-18)32-19-8-12-29(13-9-19)16-22(23(24,25)26)10-2-3-11-22/h14-15,17-19H,2-13,16H2,1H3/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106218

(CHEMBL3598111)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC3(F)CCCC3)CC2)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-2.65,7.7,;-1.31,8.47,;-1.3,9.7,;-1.21,6.97,;.29,6.64,;1.07,7.97,;.05,9.12,;-1.32,5.39,;-1.33,3.85,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C22H34FN3O4S/c1-31(27,28)21-15-24-20(14-25-21)30-18-6-4-17(5-7-18)29-19-8-12-26(13-9-19)16-22(23)10-2-3-11-22/h14-15,17-19H,2-13,16H2,1H3/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 291 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106152

(CHEMBL3598083)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cc1F)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;5.34,-2.76,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H34FNO6S/c1-23(2,3)31-22(26)25-13-11-18(12-14-25)29-16-5-7-17(8-6-16)30-21-10-9-19(15-20(21)24)32(4,27)28/h9-10,15-18H,5-8,11-14H2,1-4H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106154

(CHEMBL3598085)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C#N |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,)| Show InChI InChI=1S/C23H32N2O4/c1-23(2,3)29-22(26)25-14-12-21(13-15-25)28-20-10-8-19(9-11-20)27-18-6-4-17(16-24)5-7-18/h4-7,19-21H,8-15H2,1-3H3/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 334 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106159

(CHEMBL3598090)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C21H33N3O6S/c1-21(2,3)30-20(25)24-11-9-17(10-12-24)28-15-5-7-16(8-6-15)29-18-13-23-19(14-22-18)31(4,26)27/h13-17H,5-12H2,1-4H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106161

(CHEMBL3598092)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)C#N |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,)| Show InChI InChI=1S/C21H30N4O4/c1-21(2,3)29-20(26)25-10-8-18(9-11-25)27-16-4-6-17(7-5-16)28-19-14-23-15(12-22)13-24-19/h13-14,16-18H,4-11H2,1-3H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 144 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106163

(CHEMBL3598094)Show SMILES CC(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:16.20,wD:13.13,(-.23,10.62,;-1.3,10.01,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C20H31N3O6S/c1-14(2)27-20(24)23-10-8-17(9-11-23)28-15-4-6-16(7-5-15)29-18-12-22-19(13-21-18)30(3,25)26/h12-17H,4-11H2,1-3H3/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106206

(CHEMBL3598099)Show SMILES C[C@@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,wD:12.12,1.0,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106207

(CHEMBL3598100)Show SMILES C[C@H](OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O)C(F)(F)F |r,wU:15.19,1.0,wD:12.12,(-2.36,10.63,;-1.3,10.01,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,)| Show InChI InChI=1S/C20H28F3N3O6S/c1-13(20(21,22)23)30-19(27)26-9-7-16(8-10-26)31-14-3-5-15(6-4-14)32-17-11-25-18(12-24-17)33(2,28)29/h11-16H,3-10H2,1-2H3/t13-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106208

(CHEMBL3598101)Show SMILES CS(=O)(=O)c1cnc(O[C@H]2CC[C@@H](CC2)OC2CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)cn1 |r,wU:9.8,wD:12.15,(9.06,2.18,;8,1.56,;9.07,.95,;7.99,2.79,;6.67,.78,;6.67,-.76,;5.34,-1.53,;4,-.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-2.66,3.08,;-4,3.86,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.65,7.7,;-3.71,8.33,;-1.31,8.47,;-1.3,10.01,;.04,10.77,;.05,12.01,;1.1,10.15,;1.11,11.38,;-2.63,10.78,;-3.7,10.17,;-2.63,12.02,;-3.7,11.4,;4,.77,;5.33,1.55,)| Show InChI InChI=1S/C20H25F6N3O6S/c1-36(31,32)16-11-27-15(10-28-16)34-13-4-2-12(3-5-13)33-14-6-8-29(9-7-14)18(30)35-17(19(21,22)23)20(24,25)26/h10-14,17H,2-9H2,1H3/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106211

(CHEMBL3598104)Show SMILES CCC(F)(F)C(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1cnc(cn1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.31,8.47,;-.24,9.08,;-.24,7.85,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C20H29F2N3O5S/c1-3-20(21,22)19(26)25-10-8-16(9-11-25)29-14-4-6-15(7-5-14)30-17-12-24-18(13-23-17)31(2,27)28/h12-16H,3-11H2,1-2H3/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106124

(CHEMBL3598112)Show SMILES CC(C)c1noc(CN2CCC(CC2)O[C@H]2CC[C@@H](CC2)Oc2cnc(cn2)S(C)(=O)=O)n1 |r,wU:18.22,wD:15.15,(3.13,7.65,;2.64,8.78,;3.38,9.77,;1.11,8.96,;.35,10.3,;-1.15,9.99,;-1.31,8.47,;-2.65,7.7,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.08,7.82,)| Show InChI InChI=1S/C22H33N5O5S/c1-15(2)22-25-20(32-26-22)14-27-10-8-18(9-11-27)30-16-4-6-17(7-5-16)31-19-12-24-21(13-23-19)33(3,28)29/h12-13,15-18H,4-11,14H2,1-3H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106125
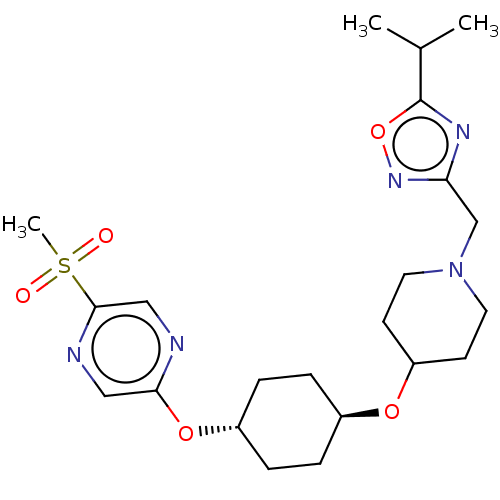
(CHEMBL3598113)Show SMILES CC(C)c1nc(CN2CCC(CC2)O[C@H]2CC[C@@H](CC2)Oc2cnc(cn2)S(C)(=O)=O)no1 |r,wU:17.21,wD:14.14,(3.13,7.65,;2.64,8.78,;3.38,9.77,;1.11,8.96,;.08,7.82,;-1.31,8.47,;-2.65,7.7,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;-1.15,9.99,;.35,10.3,)| Show InChI InChI=1S/C22H33N5O5S/c1-15(2)22-25-19(26-32-22)14-27-10-8-18(9-11-27)30-16-4-6-17(7-5-16)31-20-12-24-21(13-23-20)33(3,28)29/h12-13,15-18H,4-11,14H2,1-3H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 56 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106126

(CHEMBL3598114)Show SMILES CC(F)Cc1noc(CN2CCC(CC2)O[C@H]2CC[C@@H](CC2)Oc2cnc(cn2)S(C)(=O)=O)n1 |r,wU:19.23,wD:16.16,(2.52,6.38,;3.26,7.37,;4.48,7.23,;2.64,8.78,;1.11,8.96,;.35,10.3,;-1.15,9.99,;-1.31,8.47,;-2.65,7.7,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;.08,7.82,)| Show InChI InChI=1S/C22H32FN5O5S/c1-15(23)11-19-26-21(33-27-19)14-28-9-7-18(8-10-28)31-16-3-5-17(6-4-16)32-20-12-25-22(13-24-20)34(2,29)30/h12-13,15-18H,3-11,14H2,1-2H3/t15?,16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50106127
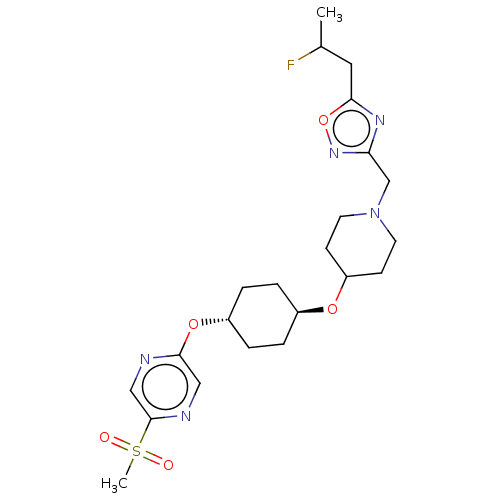
(CHEMBL3598115)Show SMILES CC(F)Cc1nc(CN2CCC(CC2)O[C@H]2CC[C@@H](CC2)Oc2cnc(cn2)S(C)(=O)=O)no1 |r,wU:18.22,wD:15.15,(2.52,6.38,;3.26,7.37,;4.48,7.23,;2.64,8.78,;1.11,8.96,;.08,7.82,;-1.31,8.47,;-2.65,7.7,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.55,;6.67,.78,;6.67,-.76,;5.34,-1.53,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,;-1.15,9.99,;.35,10.3,)| Show InChI InChI=1S/C22H32FN5O5S/c1-15(23)11-20-26-19(27-33-20)14-28-9-7-18(8-10-28)31-16-3-5-17(6-4-16)32-21-12-25-22(13-24-21)34(2,29)30/h12-13,15-18H,3-11,14H2,1-2H3/t15?,16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 276 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 by serum shift assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50364559

(CHEMBL1951032)Show SMILES COc1c(Nc2ccc(nc2C)S(C)(=O)=O)ncnc1OC1CCN(CC1)C(=O)OC(C)C Show InChI InChI=1S/C21H29N5O6S/c1-13(2)31-21(27)26-10-8-15(9-11-26)32-20-18(30-4)19(22-12-23-20)25-16-6-7-17(24-14(16)3)33(5,28)29/h6-7,12-13,15H,8-11H2,1-5H3,(H,22,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 421 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50056004
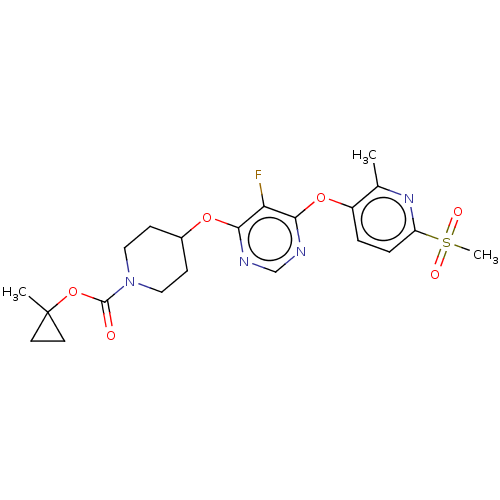
(CHEMBL3326667)Show SMILES Cc1nc(ccc1Oc1ncnc(OC2CCN(CC2)C(=O)OC2(C)CC2)c1F)S(C)(=O)=O Show InChI InChI=1S/C21H25FN4O6S/c1-13-15(4-5-16(25-13)33(3,28)29)31-19-17(22)18(23-12-24-19)30-14-6-10-26(11-7-14)20(27)32-21(2)8-9-21/h4-5,12,14H,6-11H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 169 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50106150

(CHEMBL3598081)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)S(C)(=O)=O |r,wU:17.21,wD:14.14,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H35NO6S/c1-23(2,3)30-22(25)24-15-13-20(14-16-24)29-18-7-5-17(6-8-18)28-19-9-11-21(12-10-19)31(4,26)27/h9-12,17-18,20H,5-8,13-16H2,1-4H3/t17-,18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50106151

(CHEMBL3598082)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)O[C@H]1CC[C@H](CC1)Oc1ccc(cc1)S(C)(=O)=O |r,wU:14.14,17.21,(-.23,10.62,;-1.3,10.01,;-1.29,11.24,;-2.36,10.63,;-1.31,8.47,;-2.65,7.7,;-3.71,8.33,;-2.65,6.16,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.33,3.85,;-1.32,5.39,;-2.67,1.54,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;2.67,-1.54,;4,-.77,;5.34,-1.53,;6.67,-.76,;6.67,.78,;5.33,1.55,;4,.77,;8,1.56,;9.06,2.18,;9.07,.95,;7.99,2.79,)| Show InChI InChI=1S/C23H35NO6S/c1-23(2,3)30-22(25)24-15-13-20(14-16-24)29-18-7-5-17(6-8-18)28-19-9-11-21(12-10-19)31(4,26)27/h9-12,17-18,20H,5-8,13-16H2,1-4H3/t17-,18+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR119 by HTRF cAMP assay |
Bioorg Med Chem Lett 25: 3034-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.102
BindingDB Entry DOI: 10.7270/Q2SB47JS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data