

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088564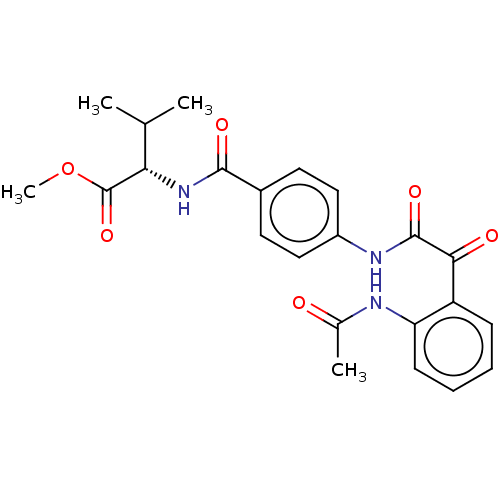 (CHEMBL3577041) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088560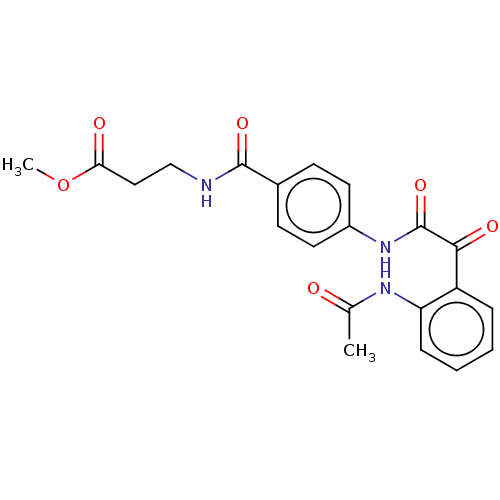 (CHEMBL3577045) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088568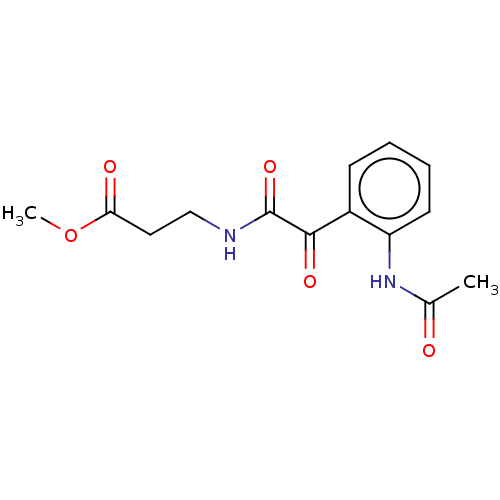 (CHEMBL3577037) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088563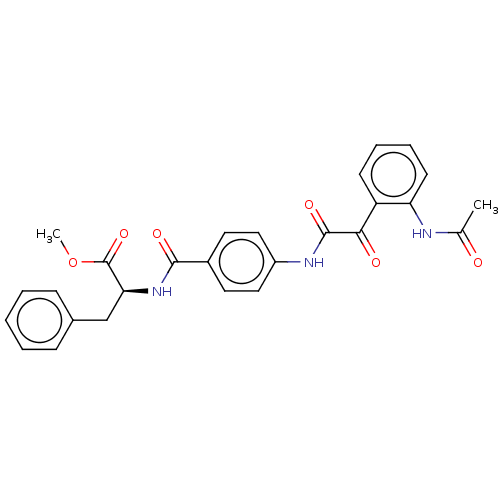 (CHEMBL3577042) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088565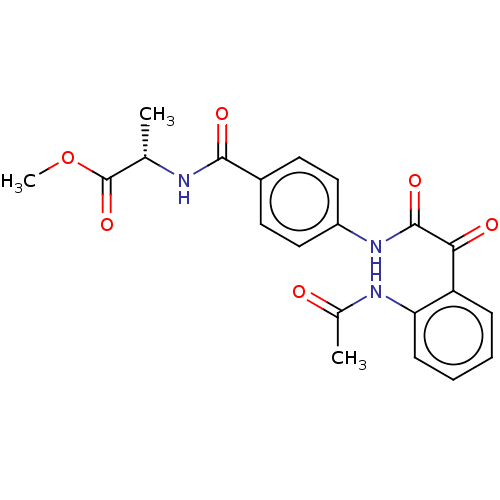 (CHEMBL3577040) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088562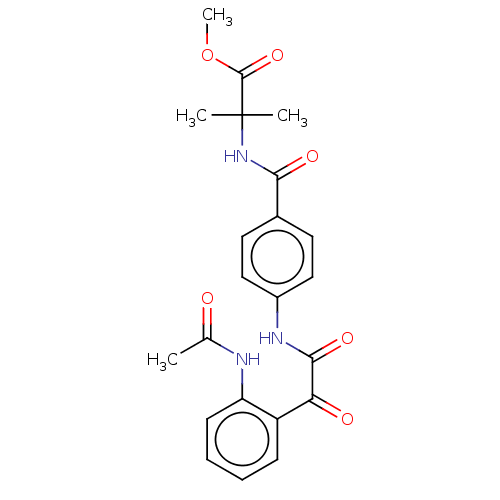 (CHEMBL3577043) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088557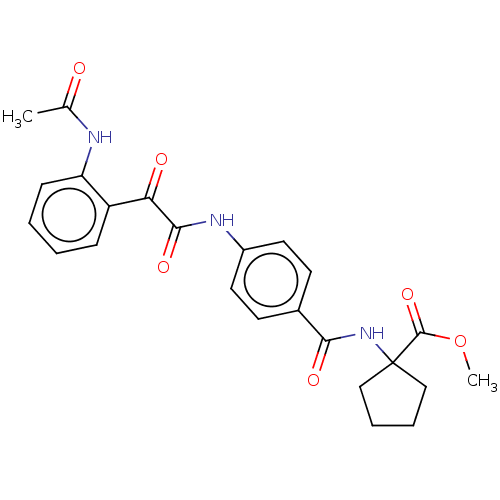 (CHEMBL3577048) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088559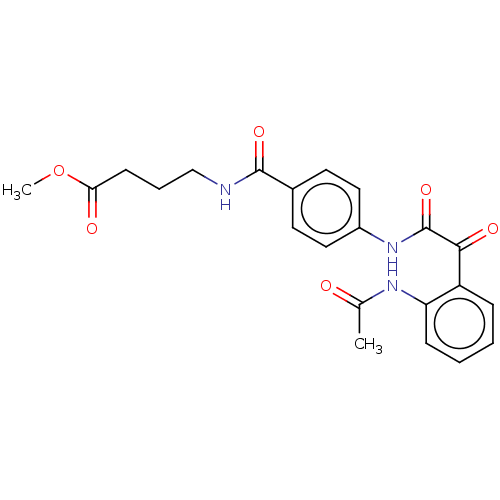 (CHEMBL3577046) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM22787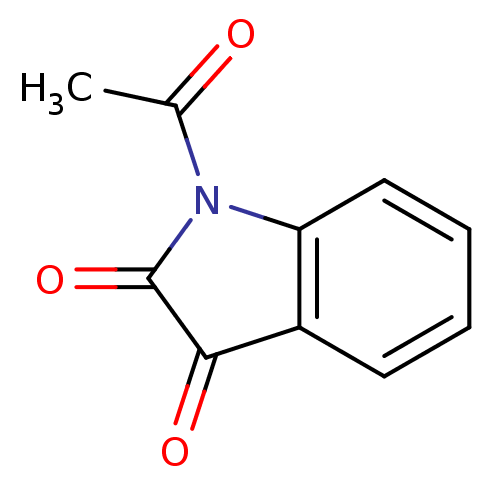 (1-acetyl-2,3-dihydro-1H-indole-2,3-dione | Acetyli...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088554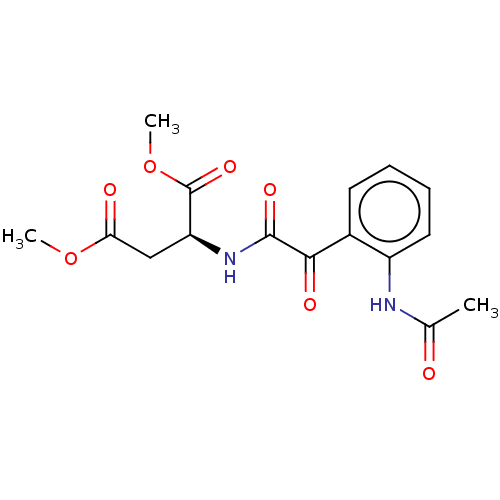 (CHEMBL3577036) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088561 (CHEMBL3577044) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088567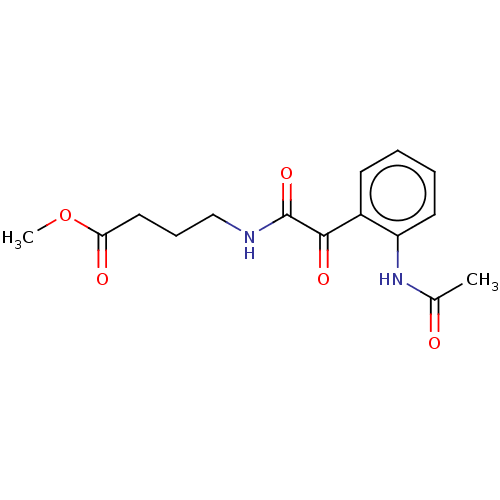 (CHEMBL3577038) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088566 (CHEMBL3577039) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088555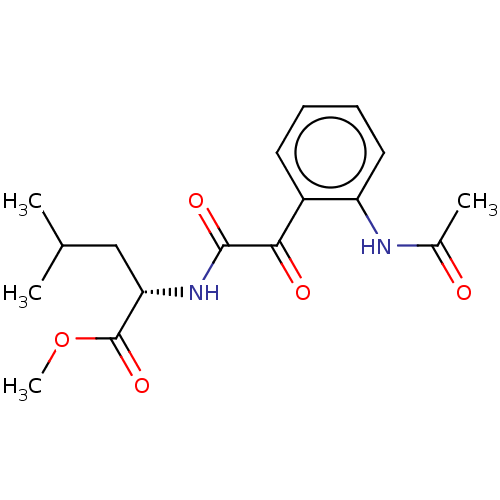 (CHEMBL3577035) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50088556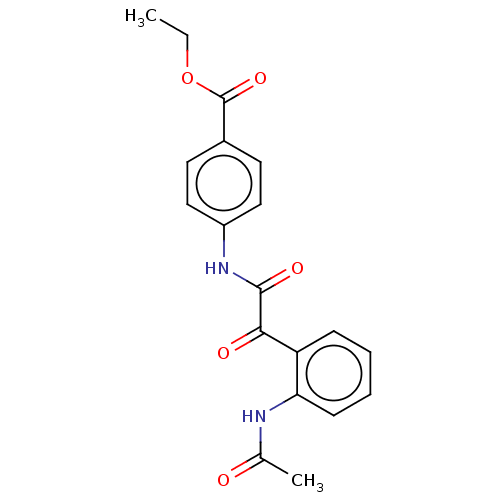 (CHEMBL3577034) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using serotonin as substrate preincubated with enzyme for 60 mins prior to substrate addition | Bioorg Med Chem Lett 25: 70-4 (2015) Article DOI: 10.1016/j.bmcl.2014.11.007 BindingDB Entry DOI: 10.7270/Q24F1SGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226429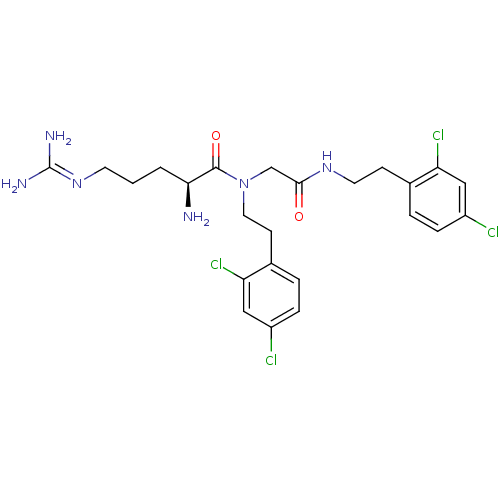 (CHEMBL237354 | [L-arginyl]-N-(2,4-dichlorophenethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226436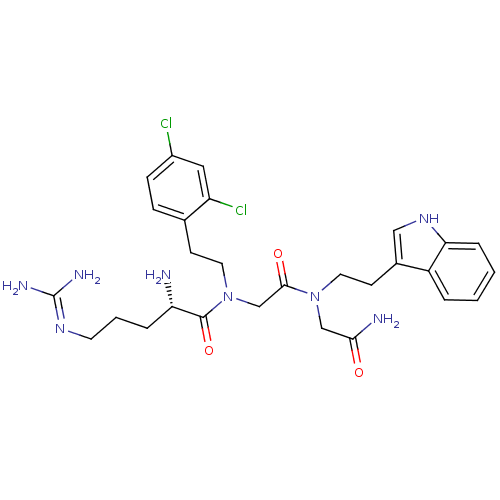 (CHEMBL401228 | [L-arginyl]-[N-(2,4-dichloropheneth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50246740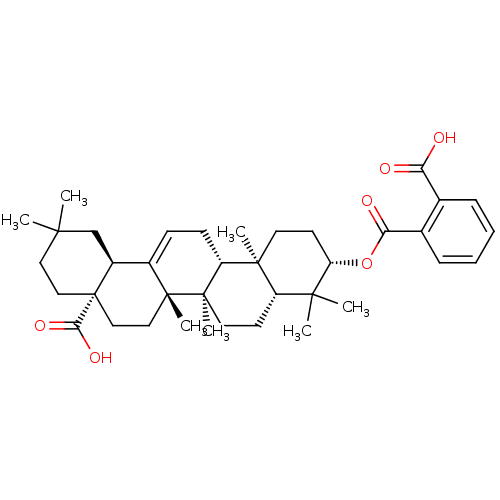 ((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-(2-carboxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496372 (CHEMBL3126478) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226431 (CHEMBL237138 | [[N-(2,4-dichlorophenethyl)-N-(6-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226430 (CHEMBL400008 | [L-arginyl]-[N-[2-(indol-3-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50277089 (CHEMBL320364 | Oleanolic Acid 3-O-Glutarate) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496355 (CHEMBL318169) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226432 (CHEMBL430173 | cis-(L-arginyl)amino-N-(2,4-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226435 ((S)-N-(2,4-dichlorophenethyl)-N-(2-((2,4-dichlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Rattus norvegicus (Rat)-RAT) | BDBM50226429 (CHEMBL237354 | [L-arginyl]-N-(2,4-dichlorophenethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of L-glutamate/glycine-activated rat NR1/NR2A NMDA receptor expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496359 (CHEMBL3126474) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226434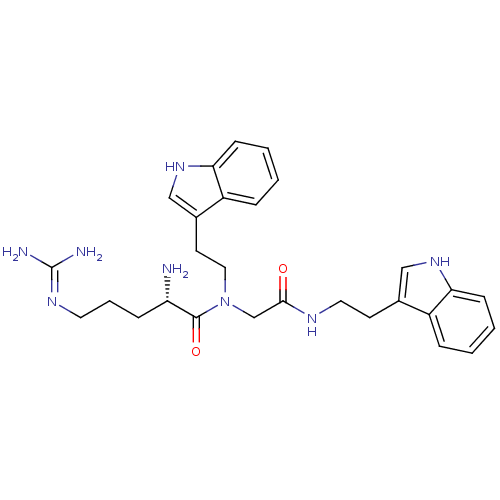 (CHEMBL392540 | [L-arginyl]-N-[2-(indol-3-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50226433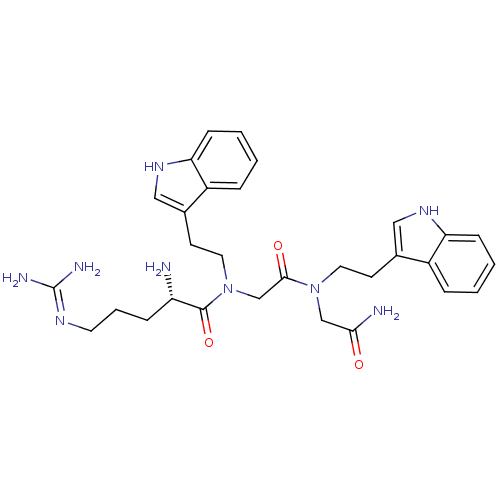 (CHEMBL395124 | [L-arginyl]-[N-[2-(indol-3-yl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of capsaicin-activated rat TRPV1 channel expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496357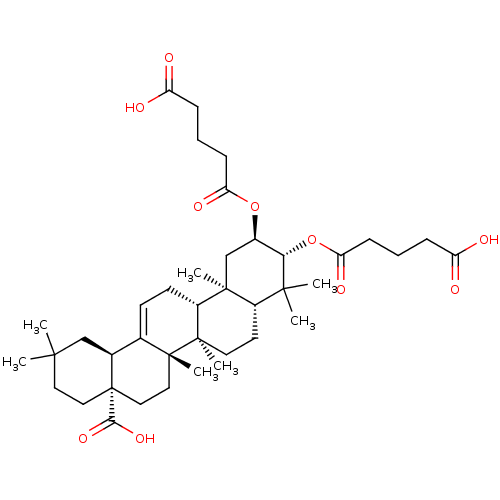 (CHEMBL3126455) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496366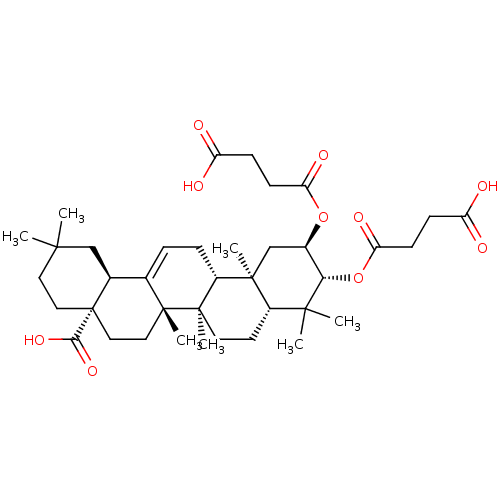 (CHEMBL3126468) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496362 (CHEMBL3126461) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162761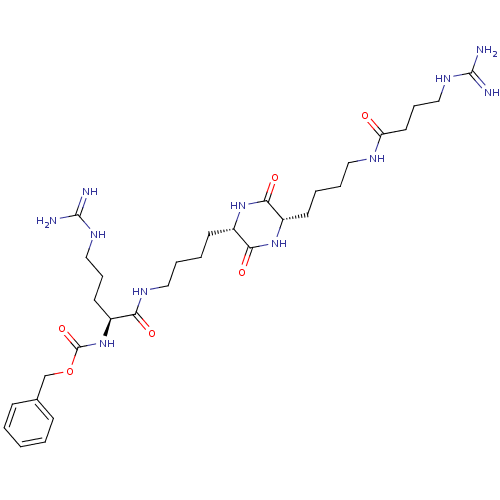 (CHEMBL180302 | [4-Guanidino-1-((S)-4-{(S)-5-[4-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496367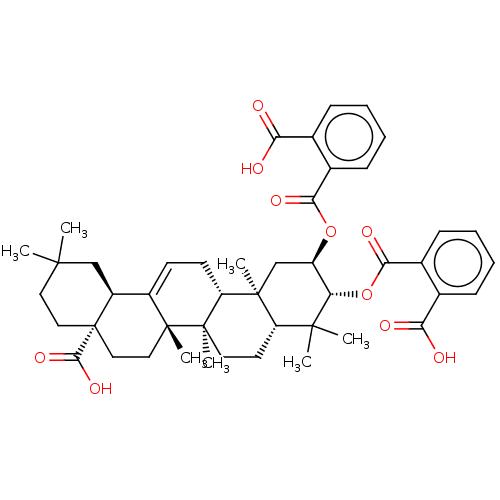 (CHEMBL3126466) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162770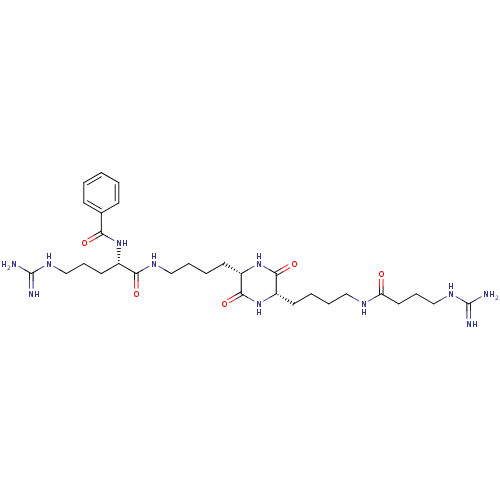 (CHEMBL180281 | N-[4-Guanidino-1-((S)-4-{(S)-5-[4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Rattus norvegicus (Rat)-RAT) | BDBM50226431 (CHEMBL237138 | [[N-(2,4-dichlorophenethyl)-N-(6-am...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of L-glutamate/glycine-activated rat NR1/NR2A NMDA receptor expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496365 (CHEMBL3126454) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50102741 (3-O-Acetyloleanolic Acid | Acetyl Oleanolic Acid) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496371 (CHEMBL3126467) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496360 (CHEMBL3126453) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162764 (CHEMBL359680 | [1-((S)-4-{(S)-5-[4-((S)-(S)-2-Benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162760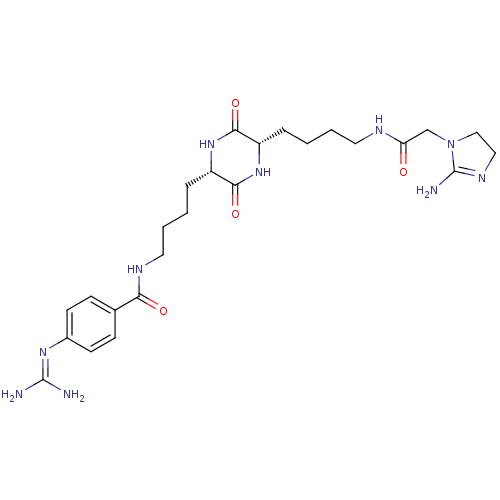 (4-Guanidino-N-[(S)-4-((S)-5-{4-[2-(2-imino-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162758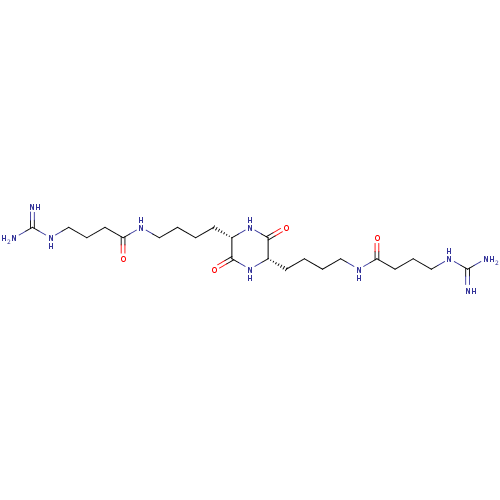 (4-Guanidino-N-(4-{(S)-5-[4-((S)-4-guanidino-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50496361 (CHEMBL3126463) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease expressed in Escherichia coli using Abz-Ala-Arg-Val-Nle-Tyr(NO2)-Glu-Ala-Nle-NH2 as substrate by FRET assay | Eur J Med Chem 74: 278-301 (2014) Article DOI: 10.1016/j.ejmech.2013.12.049 BindingDB Entry DOI: 10.7270/Q2T43X2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162762 (CHEMBL181327 | [(S)-4-Guanidino-1-((S)-4-{(S)-5-[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162763 (CHEMBL181685 | N-((S)-4-{5-[4-(3-Guanidino-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Rattus norvegicus (Rat)-RAT) | BDBM50226432 (CHEMBL430173 | cis-(L-arginyl)amino-N-(2,4-dichlor...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Blockade of L-glutamate/glycine-activated rat NR1/NR2A NMDA receptor expressed in Xenopus oocytes | J Med Chem 50: 6133-43 (2007) Article DOI: 10.1021/jm070612v BindingDB Entry DOI: 10.7270/Q2FQ9WC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162759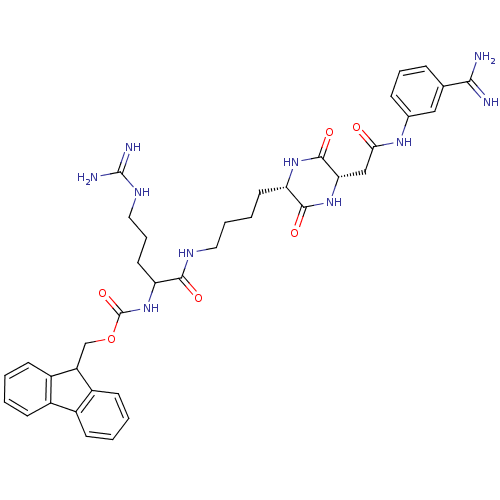 (CHEMBL178315 | [1-(4-{(S)-5-[((S)-3-Carbamimidoyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50162765 ((S)-N-[4-Guanidino-1-((S)-4-{(S)-5-[4-(5-guanidino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibitory concentration against tryptase | Bioorg Med Chem Lett 15: 1659-64 (2005) Article DOI: 10.1016/j.bmcl.2005.01.048 BindingDB Entry DOI: 10.7270/Q21N80N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |