 Found 44 hits with Last Name = 'alley' and Initial = 'gm'
Found 44 hits with Last Name = 'alley' and Initial = 'gm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM10616

((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...)Show InChI InChI=1S/C15H20N2O3/c1-4-16-14(18)20-10-5-6-12-11(9-10)15(2)7-8-19-13(15)17(12)3/h5-6,9,13H,4,7-8H2,1-3H3,(H,16,18)/t13?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10614
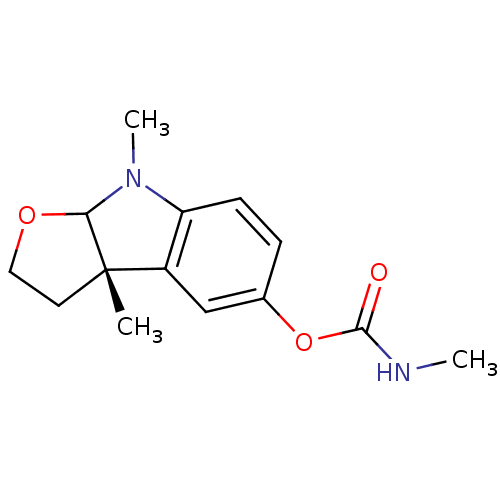
((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...)Show InChI InChI=1S/C14H18N2O3/c1-14-6-7-18-12(14)16(3)11-5-4-9(8-10(11)14)19-13(17)15-2/h4-5,8,12H,6-7H2,1-3H3,(H,15,17)/t12?,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10610

((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...)Show SMILES CCNC(=O)Oc1ccc2N(C)C3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C16H23N3O2/c1-5-17-15(20)21-11-6-7-13-12(10-11)16(2)8-9-18(3)14(16)19(13)4/h6-7,10,14H,5,8-9H2,1-4H3,(H,17,20)/t14?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10972
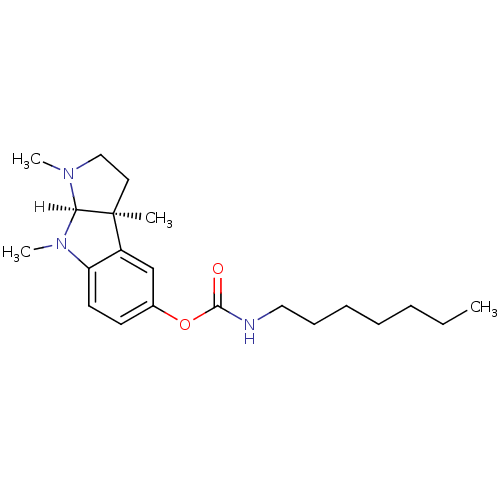
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50161789

(CHEMBL369554 | Ethyl-carbamic acid 3a-methyl-2,3,3...)Show InChI InChI=1S/C14H17NO4/c1-3-15-13(16)18-9-4-5-11-10(8-9)14(2)6-7-17-12(14)19-11/h4-5,8,12H,3,6-7H2,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10611
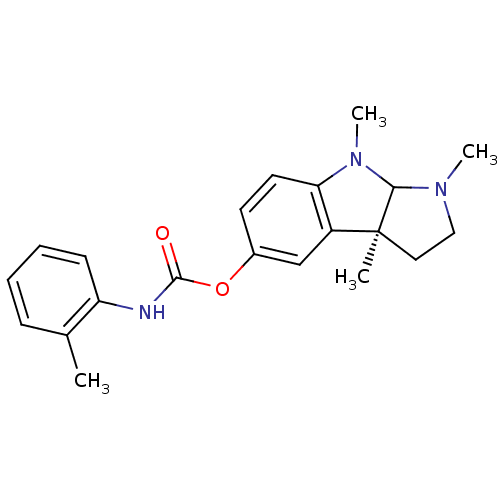
((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...)Show SMILES CN1CC[C@]2(C)C1N(C)c1ccc(OC(=O)Nc3ccccc3C)cc21 |r| Show InChI InChI=1S/C21H25N3O2/c1-14-7-5-6-8-17(14)22-20(25)26-15-9-10-18-16(13-15)21(2)11-12-23(3)19(21)24(18)4/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19?,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10617

((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...)Show SMILES CN1C2OCC[C@@]2(C)c2cc(OC(=O)Nc3ccccc3C)ccc12 |r| Show InChI InChI=1S/C20H22N2O3/c1-13-6-4-5-7-16(13)21-19(23)25-14-8-9-17-15(12-14)20(2)10-11-24-18(20)22(17)3/h4-9,12,18H,10-11H2,1-3H3,(H,21,23)/t18?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10619
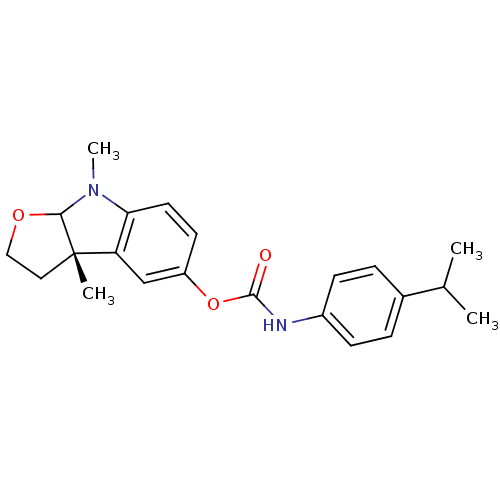
((4-Isopropyl-phenyl)-carbamic acid (S)-3a,8-dimeth...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)C4OCC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C22H26N2O3/c1-14(2)15-5-7-16(8-6-15)23-21(25)27-17-9-10-19-18(13-17)22(3)11-12-26-20(22)24(19)4/h5-10,13-14,20H,11-12H2,1-4H3,(H,23,25)/t20?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50161786

((4-Isopropyl-phenyl)-carbamic acid 1-methyl-8,10-d...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3OC4CC(C)(CO4)c3c2)cc1 Show InChI InChI=1S/C21H23NO4/c1-13(2)14-4-6-15(7-5-14)22-20(23)25-16-8-9-18-17(10-16)21(3)11-19(26-18)24-12-21/h4-10,13,19H,11-12H2,1-3H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10958
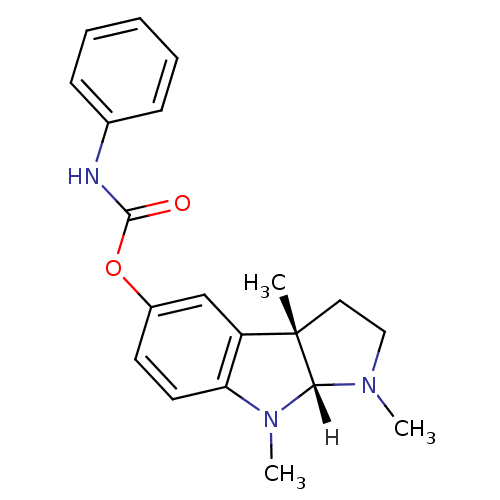
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161787
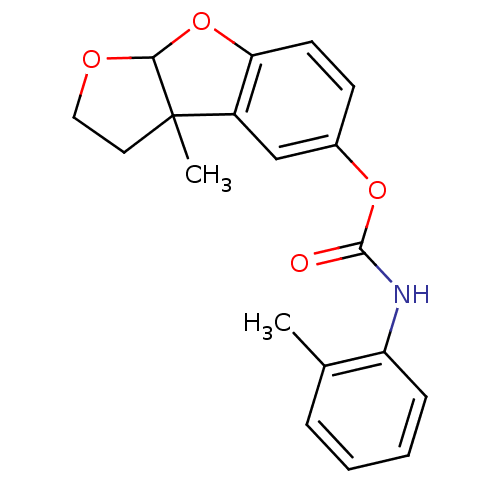
(CHEMBL178770 | o-Tolyl-carbamic acid 3a-methyl-2,3...)Show InChI InChI=1S/C19H19NO4/c1-12-5-3-4-6-15(12)20-18(21)23-13-7-8-16-14(11-13)19(2)9-10-22-17(19)24-16/h3-8,11,17H,9-10H2,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50161790
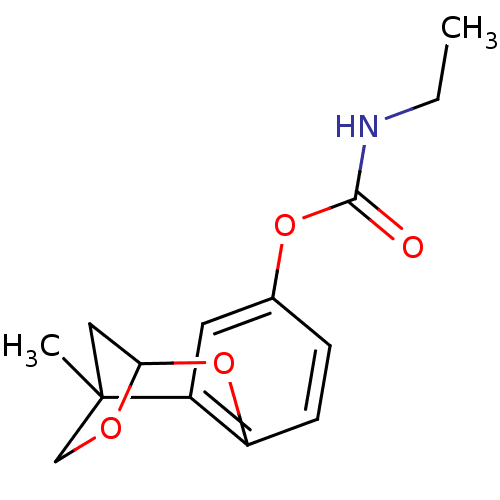
(CHEMBL362704 | Ethyl-carbamic acid 1-methyl-8,10-d...)Show InChI InChI=1S/C14H17NO4/c1-3-15-13(16)18-9-4-5-11-10(6-9)14(2)7-12(19-11)17-8-14/h4-6,12H,3,7-8H2,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50161785
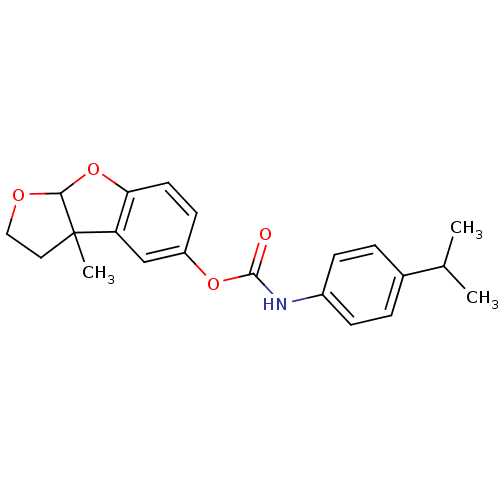
((4-Isopropyl-phenyl)-carbamic acid 3a-methyl-2,3,3...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3OC4OCCC4(C)c3c2)cc1 Show InChI InChI=1S/C21H23NO4/c1-13(2)14-4-6-15(7-5-14)22-20(23)25-16-8-9-18-17(12-16)21(3)10-11-24-19(21)26-18/h4-9,12-13,19H,10-11H2,1-3H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10614

((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...)Show InChI InChI=1S/C14H18N2O3/c1-14-6-7-18-12(14)16(3)11-5-4-9(8-10(11)14)19-13(17)15-2/h4-5,8,12H,6-7H2,1-3H3,(H,15,17)/t12?,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10620
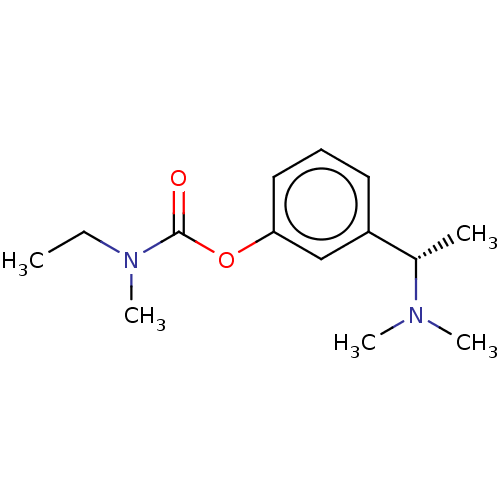
((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199522
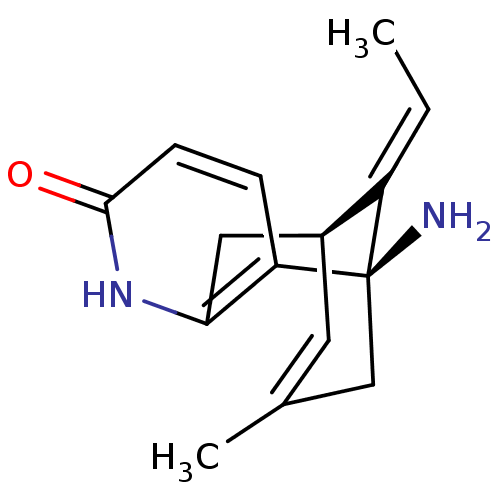
((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM10613
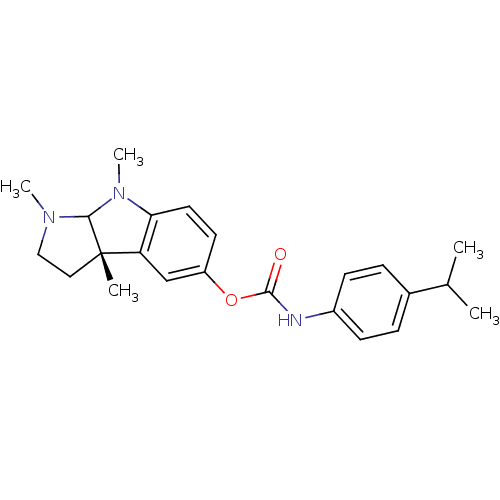
((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)C4N(C)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C23H29N3O2/c1-15(2)16-6-8-17(9-7-16)24-22(27)28-18-10-11-20-19(14-18)23(3)12-13-25(4)21(23)26(20)5/h6-11,14-15,21H,12-13H2,1-5H3,(H,24,27)/t21?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161791
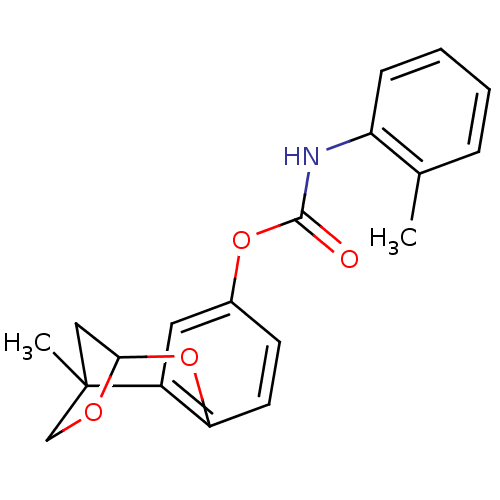
(CHEMBL178294 | o-Tolyl-carbamic acid 1-methyl-8,10...)Show InChI InChI=1S/C19H19NO4/c1-12-5-3-4-6-15(12)20-18(21)23-13-7-8-16-14(9-13)19(2)10-17(24-16)22-11-19/h3-9,17H,10-11H2,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10616

((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...)Show InChI InChI=1S/C15H20N2O3/c1-4-16-14(18)20-10-5-6-12-11(9-10)15(2)7-8-19-13(15)17(12)3/h5-6,9,13H,4,7-8H2,1-3H3,(H,16,18)/t13?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10610

((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...)Show SMILES CCNC(=O)Oc1ccc2N(C)C3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C16H23N3O2/c1-5-17-15(20)21-11-6-7-13-12(10-11)16(2)8-9-18(3)14(16)19(13)4/h6-7,10,14H,5,8-9H2,1-4H3,(H,17,20)/t14?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM11686
((-)-O-[(2-ethylphenyl)carbamoyl]geneseroline | (3a...)Show SMILES [H][C@@]12N(C)c3ccc(OC(=O)Nc4ccccc4CC)cc3[C@]1(C)CC[N+]2(C)[O-] |r| Show InChI InChI=1S/C22H27N3O3/c1-5-15-8-6-7-9-18(15)23-21(26)28-16-10-11-19-17(14-16)22(2)12-13-25(4,27)20(22)24(19)3/h6-11,14,20H,5,12-13H2,1-4H3,(H,23,26)/t20-,22-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161789

(CHEMBL369554 | Ethyl-carbamic acid 3a-methyl-2,3,3...)Show InChI InChI=1S/C14H17NO4/c1-3-15-13(16)18-9-4-5-11-10(8-9)14(2)6-7-17-12(14)19-11/h4-5,8,12H,3,6-7H2,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10613

((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)C4N(C)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C23H29N3O2/c1-15(2)16-6-8-17(9-7-16)24-22(27)28-18-10-11-20-19(14-18)23(3)12-13-25(4)21(23)26(20)5/h6-11,14-15,21H,12-13H2,1-5H3,(H,24,27)/t21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161790

(CHEMBL362704 | Ethyl-carbamic acid 1-methyl-8,10-d...)Show InChI InChI=1S/C14H17NO4/c1-3-15-13(16)18-9-4-5-11-10(6-9)14(2)7-12(19-11)17-8-14/h4-6,12H,3,7-8H2,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10958

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10617

((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...)Show SMILES CN1C2OCC[C@@]2(C)c2cc(OC(=O)Nc3ccccc3C)ccc12 |r| Show InChI InChI=1S/C20H22N2O3/c1-13-6-4-5-7-16(13)21-19(23)25-14-8-9-17-15(12-14)20(2)10-11-24-18(20)22(17)3/h4-9,12,18H,10-11H2,1-3H3,(H,21,23)/t18?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM11686

((-)-O-[(2-ethylphenyl)carbamoyl]geneseroline | (3a...)Show SMILES [H][C@@]12N(C)c3ccc(OC(=O)Nc4ccccc4CC)cc3[C@]1(C)CC[N+]2(C)[O-] |r| Show InChI InChI=1S/C22H27N3O3/c1-5-15-8-6-7-9-18(15)23-21(26)28-16-10-11-19-17(14-16)22(2)12-13-25(4,27)20(22)24(19)3/h6-11,14,20H,5,12-13H2,1-4H3,(H,23,26)/t20-,22-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10611

((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...)Show SMILES CN1CC[C@]2(C)C1N(C)c1ccc(OC(=O)Nc3ccccc3C)cc21 |r| Show InChI InChI=1S/C21H25N3O2/c1-14-7-5-6-8-17(14)22-20(25)26-15-9-10-18-16(13-15)21(2)11-12-23(3)19(21)24(18)4/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161785

((4-Isopropyl-phenyl)-carbamic acid 3a-methyl-2,3,3...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3OC4OCCC4(C)c3c2)cc1 Show InChI InChI=1S/C21H23NO4/c1-13(2)14-4-6-15(7-5-14)22-20(23)25-16-8-9-18-17(12-16)21(3)10-11-24-19(21)26-18/h4-9,12-13,19H,10-11H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10619

((4-Isopropyl-phenyl)-carbamic acid (S)-3a,8-dimeth...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)C4OCC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C22H26N2O3/c1-14(2)15-5-7-16(8-6-15)23-21(25)27-17-9-10-19-18(13-17)22(3)11-12-26-20(22)24(19)4/h5-10,13-14,20H,11-12H2,1-4H3,(H,23,25)/t20?,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161786

((4-Isopropyl-phenyl)-carbamic acid 1-methyl-8,10-d...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3OC4CC(C)(CO4)c3c2)cc1 Show InChI InChI=1S/C21H23NO4/c1-13(2)14-4-6-15(7-5-14)22-20(23)25-16-8-9-18-17(10-16)21(3)11-19(26-18)24-12-21/h4-10,13,19H,11-12H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human erythrocyte Acetylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50161791

(CHEMBL178294 | o-Tolyl-carbamic acid 1-methyl-8,10...)Show InChI InChI=1S/C19H19NO4/c1-12-5-3-4-6-15(12)20-18(21)23-13-7-8-16-14(9-13)19(2)10-17(24-16)22-11-19/h3-9,17H,10-11H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50161787

(CHEMBL178770 | o-Tolyl-carbamic acid 3a-methyl-2,3...)Show InChI InChI=1S/C19H19NO4/c1-12-5-3-4-6-15(12)20-18(21)23-13-7-8-16-14(11-13)19(2)9-10-22-17(19)24-16/h3-8,11,17H,9-10H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma Butyrylcholinesterase |
J Med Chem 48: 986-94 (2005)
Article DOI: 10.1021/jm049309+
BindingDB Entry DOI: 10.7270/Q2TM7BWQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data