

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50287290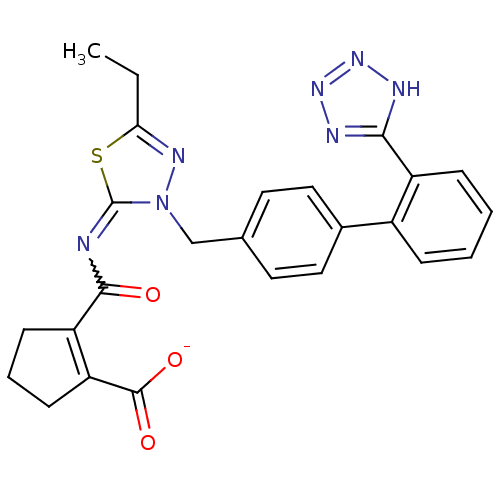 (CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287294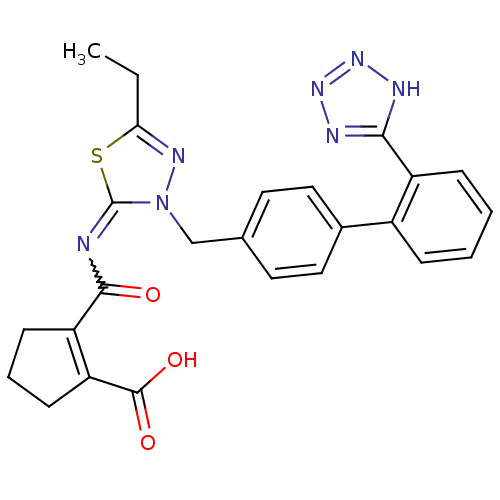 (2-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287291 (CHEMBL35381 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287289 (2-Chloro-N-[5-ethyl-3-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287292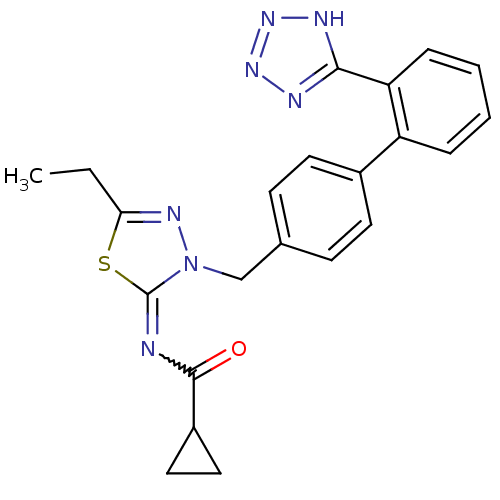 (CHEMBL34190 | Cyclopropanecarboxylic acid [5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287295 (CHEMBL35475 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50406795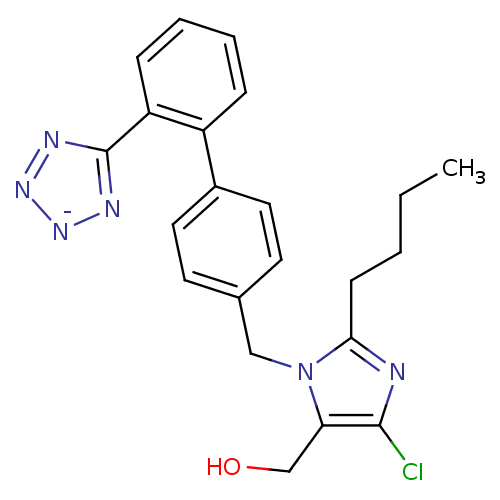 (Cozaar | LOSARTAN POTASSIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287293 (CHEMBL286537 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131703 (CHEMBL3633520) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131705 (CHEMBL3633518) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131702 (CHEMBL3633521) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131701 (CHEMBL3633522) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50366826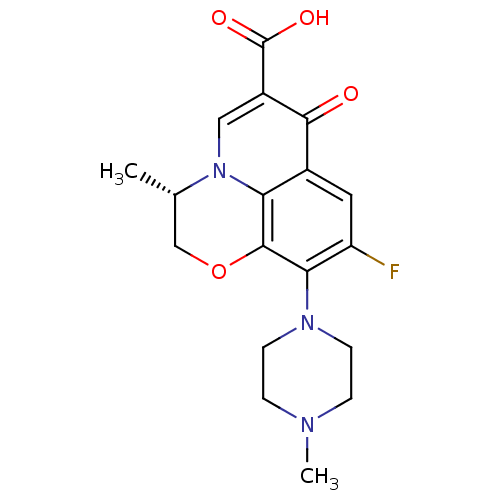 (DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131704 (CHEMBL3633519) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 553 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131700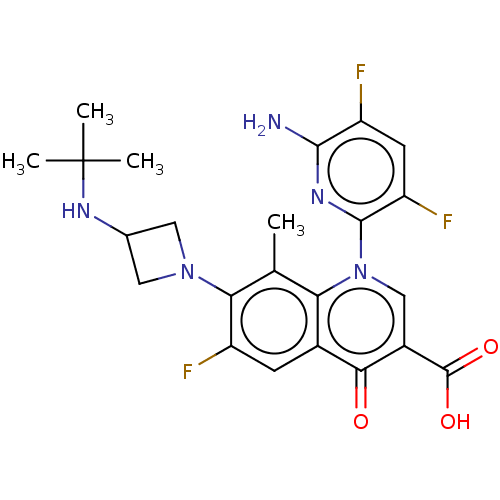 (CHEMBL3633523) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 694 | n/a | n/a | n/a | n/a | n/a | n/a |
Wakunaga Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase preincubated for 5 mins at 37 degC before addition of relaxed pBR322 as substrate by supercoiling assay | Eur J Med Chem 103: 354-60 (2015) Article DOI: 10.1016/j.ejmech.2015.08.015 BindingDB Entry DOI: 10.7270/Q22B90V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||