 Found 53545 hits with Last Name = 'ao' and Initial = 'y'
Found 53545 hits with Last Name = 'ao' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605126

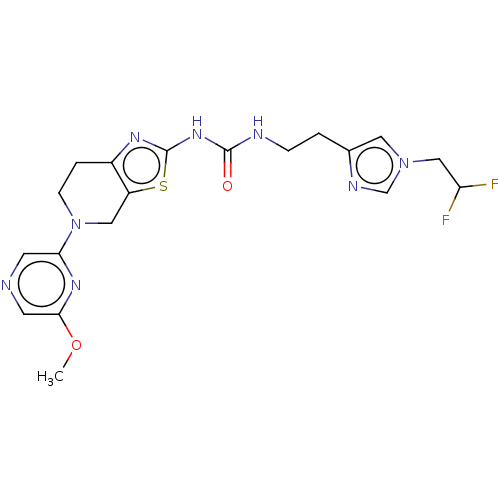
(CHEMBL5187340)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCNCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093352
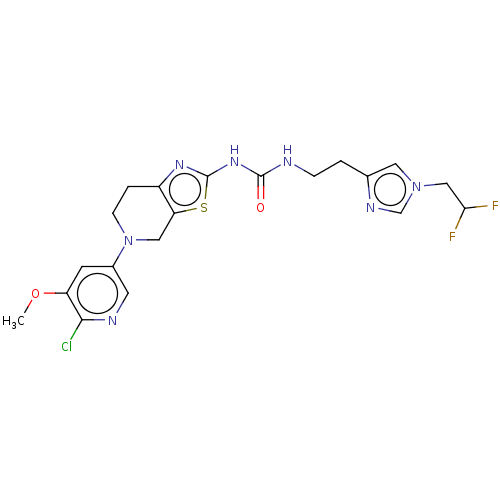
(CHEMBL3586678)Show SMILES COc1cncc(n1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C19H22F2N8O2S/c1-31-17-7-22-6-16(26-17)29-5-3-13-14(9-29)32-19(25-13)27-18(30)23-4-2-12-8-28(11-24-12)10-15(20)21/h6-8,11,15H,2-5,9-10H2,1H3,(H2,23,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093351
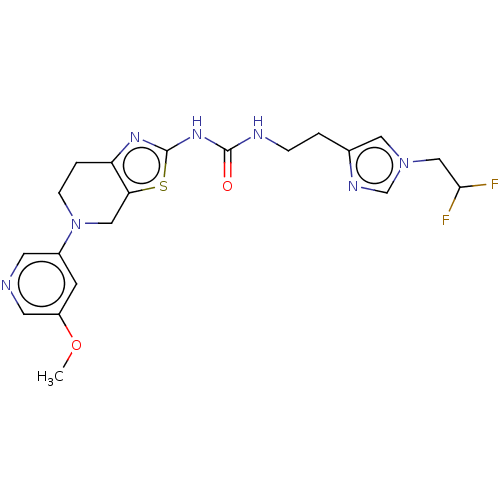
(CHEMBL3585362)Show SMILES COc1cc(cnc1Cl)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22ClF2N7O2S/c1-32-15-6-13(7-25-18(15)21)30-5-3-14-16(9-30)33-20(27-14)28-19(31)24-4-2-12-8-29(11-26-12)10-17(22)23/h6-8,11,17H,2-5,9-10H2,1H3,(H2,24,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093355
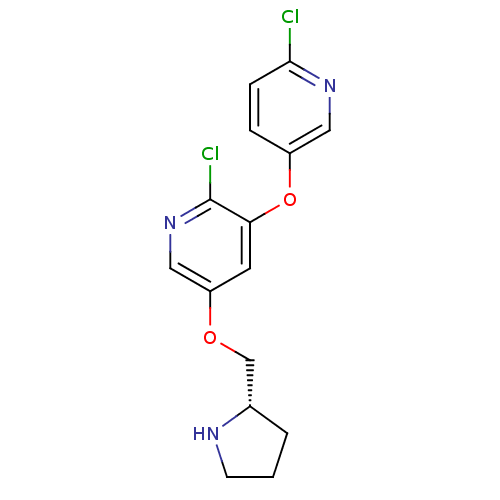
(CHEMBL3586677)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H23F2N7O2S/c1-31-15-6-14(7-23-8-15)29-5-3-16-17(10-29)32-20(26-16)27-19(30)24-4-2-13-9-28(12-25-13)11-18(21)22/h6-9,12,18H,2-5,10-11H2,1H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50295955
(5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...)Show SMILES Clc1ccc(Oc2cc(OC[C@@H]3CCCN3)cnc2Cl)cn1 |r| Show InChI InChI=1S/C15H15Cl2N3O2/c16-14-4-3-11(7-19-14)22-13-6-12(8-20-15(13)17)21-9-10-2-1-5-18-10/h3-4,6-8,10,18H,1-2,5,9H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50295955

(5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...)Show SMILES Clc1ccc(Oc2cc(OC[C@@H]3CCCN3)cnc2Cl)cn1 |r| Show InChI InChI=1S/C15H15Cl2N3O2/c16-14-4-3-11(7-19-14)22-13-6-12(8-20-15(13)17)21-9-10-2-1-5-18-10/h3-4,6-8,10,18H,1-2,5,9H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093356
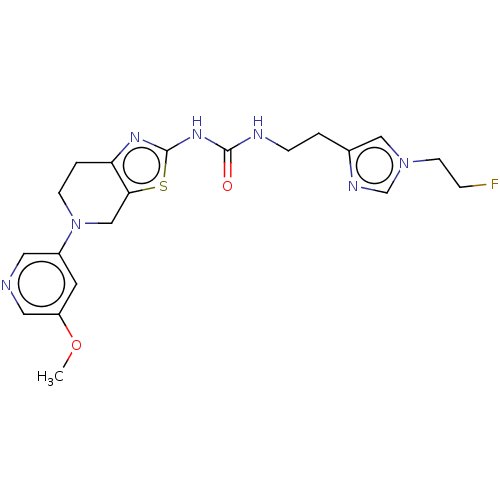
(CHEMBL3586676)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCF)cn3)sc2C1 Show InChI InChI=1S/C20H24FN7O2S/c1-30-16-8-15(9-22-10-16)28-6-3-17-18(12-28)31-20(25-17)26-19(29)23-5-2-14-11-27(7-4-21)13-24-14/h8-11,13H,2-7,12H2,1H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM209097
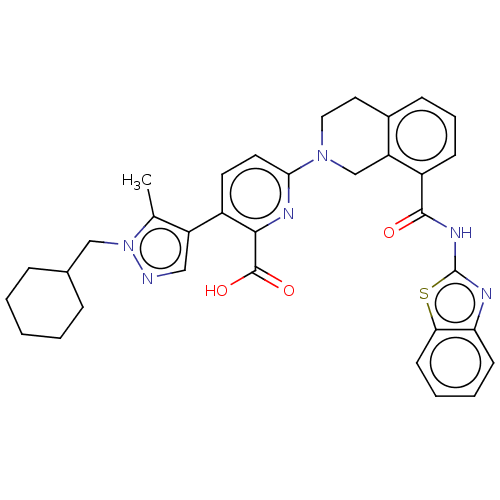
(US9266877, 43)Show SMILES Cc1c(cnn1CC1CCCCC1)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C34H34N6O3S/c1-21-26(18-35-40(21)19-22-8-3-2-4-9-22)24-14-15-30(37-31(24)33(42)43)39-17-16-23-10-7-11-25(27(23)20-39)32(41)38-34-36-28-12-5-6-13-29(28)44-34/h5-7,10-15,18,22H,2-4,8-9,16-17,19-20H2,1H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093354

(CHEMBL3586679)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22F3N7O2S/c1-32-15-6-14(7-24-8-15)30-5-3-16-17(10-30)33-19(27-16)28-18(31)25-4-2-13-9-29(12-26-13)11-20(21,22)23/h6-9,12H,2-5,10-11H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50263171
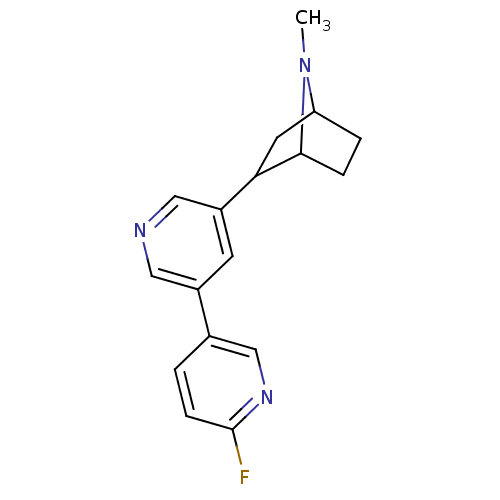
((+)-2-(6'-[18F]fluoro-3,3'-bipyridin-5-yl)-7-methy...)Show SMILES CN1C2CCC1C(C2)c1cncc(c1)-c1ccc(F)nc1 |TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-3-4-16(21)15(7-14)13-6-12(8-19-9-13)11-2-5-17(18)20-10-11/h2,5-6,8-10,14-16H,3-4,7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50263171

((+)-2-(6'-[18F]fluoro-3,3'-bipyridin-5-yl)-7-methy...)Show SMILES CN1C2CCC1C(C2)c1cncc(c1)-c1ccc(F)nc1 |TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-3-4-16(21)15(7-14)13-6-12(8-19-9-13)11-2-5-17(18)20-10-11/h2,5-6,8-10,14-16H,3-4,7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030752
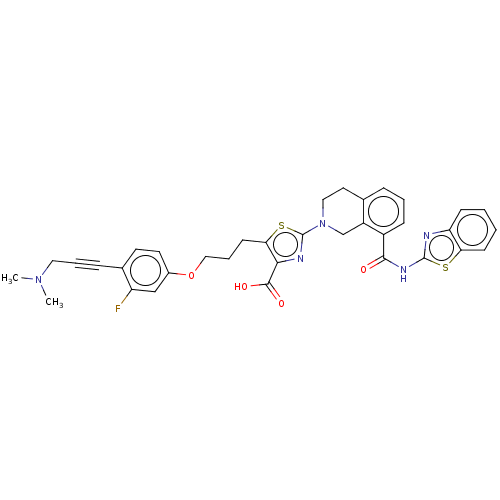
(CHEMBL3342333)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-9-23-14-15-24(20-27(23)36)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-22-8-5-10-25(26(22)21-41)32(42)39-34-37-28-11-3-4-12-29(28)46-34/h3-5,8,10-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
Eur J Med Chem 177: 63-75 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.019
BindingDB Entry DOI: 10.7270/Q2R78JMK |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757
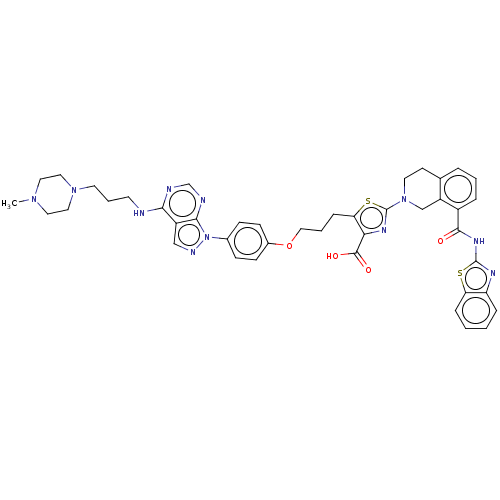
(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758
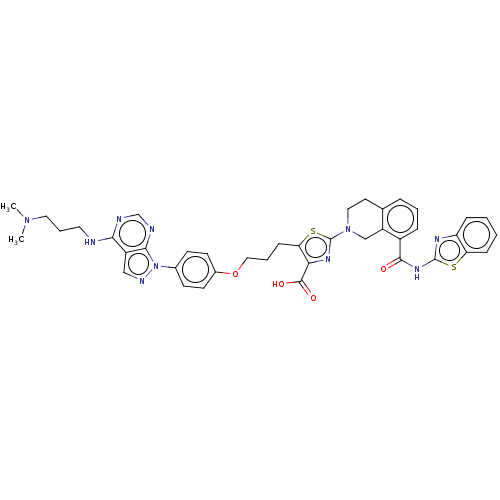
(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759
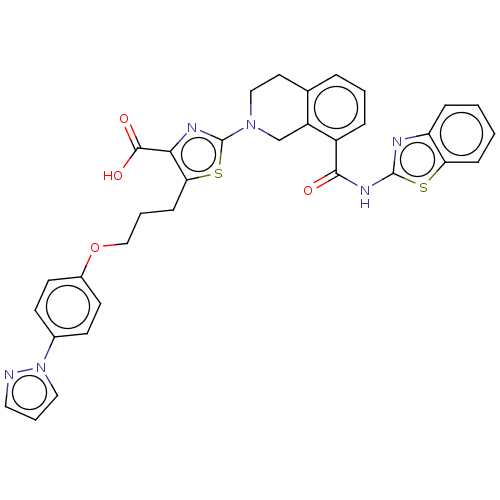
(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093395
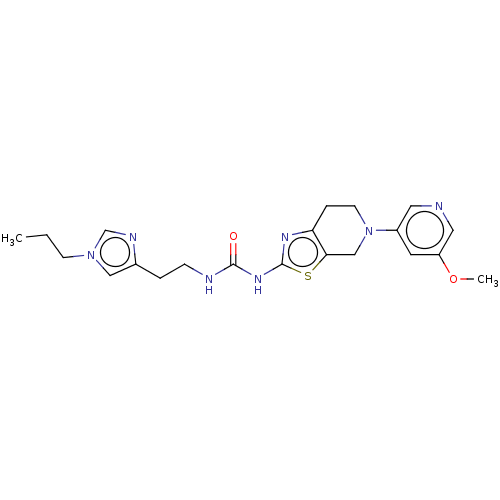
(CHEMBL3586674)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3CCN(Cc3s2)c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H27N7O2S/c1-3-7-27-12-15(24-14-27)4-6-23-20(29)26-21-25-18-5-8-28(13-19(18)31-21)16-9-17(30-2)11-22-10-16/h9-12,14H,3-8,13H2,1-2H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754
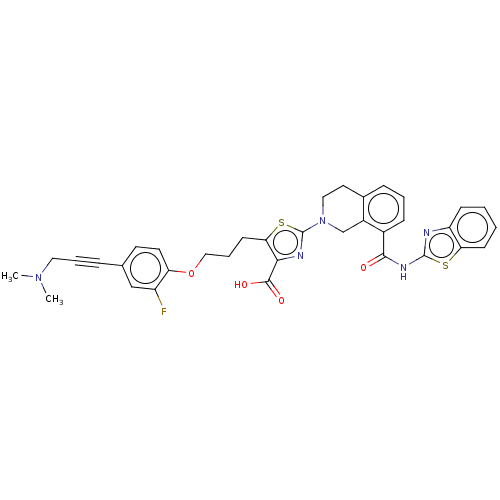
(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
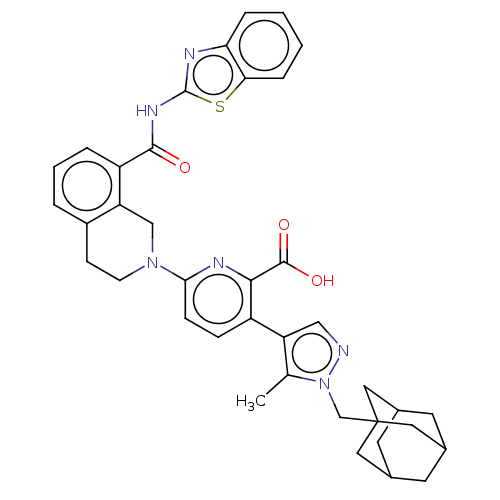
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162797
(CHEMBL3793424)Show SMILES Cc1c(cnn1CC12CC3CC(CC(C3)C1)C2)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 |TLB:14:9:16:12.13.15,14:13:16:10.9.8,THB:12:11:8:14.13.15,12:13:10.11.16:8| Show InChI InChI=1S/C38H38N6O3S/c1-22-29(19-39-44(22)21-38-16-23-13-24(17-38)15-25(14-23)18-38)27-9-10-33(41-34(27)36(46)47)43-12-11-26-5-4-6-28(30(26)20-43)35(45)42-37-40-31-7-2-3-8-32(31)48-37/h2-10,19,23-25H,11-18,20-21H2,1H3,(H,46,47)(H,40,42,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL (unknown origin) |
Eur J Med Chem 177: 63-75 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.019
BindingDB Entry DOI: 10.7270/Q2R78JMK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50561528
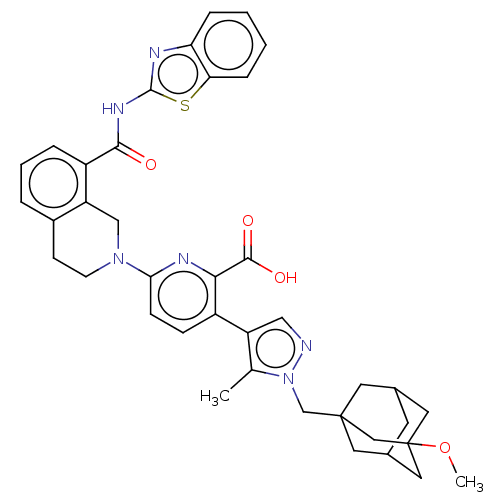
(CHEMBL4762875)Show SMILES COC12CC3CC(CC(Cn4ncc(c4C)-c4ccc(nc4C(O)=O)N4CCc5cccc(C(=O)Nc6nc7ccccc7s6)c5C4)(C3)C1)C2 |TLB:1:2:5.4.47:7,5:6:4.3.47:48,THB:5:4:6.49.7:48,3:4:7:49.2.48,3:2:5.4.47:7| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162797

(CHEMBL3793424)Show SMILES Cc1c(cnn1CC12CC3CC(CC(C3)C1)C2)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 |TLB:14:9:16:12.13.15,14:13:16:10.9.8,THB:12:11:8:14.13.15,12:13:10.11.16:8| Show InChI InChI=1S/C38H38N6O3S/c1-22-29(19-39-44(22)21-38-16-23-13-24(17-38)15-25(14-23)18-38)27-9-10-33(41-34(27)36(46)47)43-12-11-26-5-4-6-28(30(26)20-43)35(45)42-37-40-31-7-2-3-8-32(31)48-37/h2-10,19,23-25H,11-18,20-21H2,1H3,(H,46,47)(H,40,42,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50343133
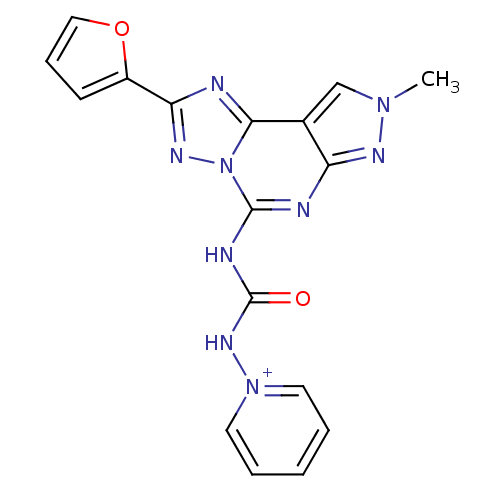
(1-(3-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1...)Show SMILES Cn1cc2c(n1)nc(NC(=O)N[n+]1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C17H13N9O2/c1-24-10-11-13(21-24)19-16(20-17(27)23-25-7-3-2-4-8-25)26-15(11)18-14(22-26)12-6-5-9-28-12/h2-10H,1H3,(H-,19,20,21,23,27)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093417
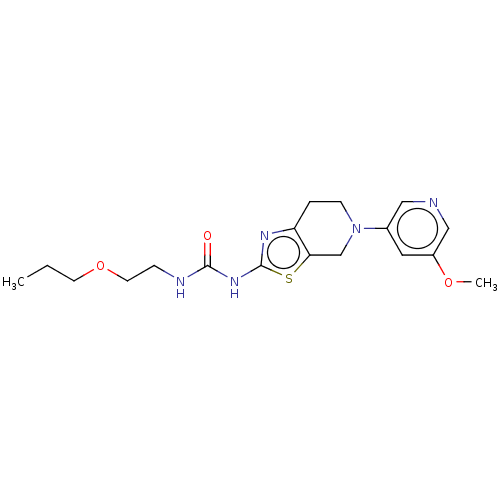
(CHEMBL3586672)Show InChI InChI=1S/C18H25N5O3S/c1-3-7-26-8-5-20-17(24)22-18-21-15-4-6-23(12-16(15)27-18)13-9-14(25-2)11-19-10-13/h9-11H,3-8,12H2,1-2H3,(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50263171

((+)-2-(6'-[18F]fluoro-3,3'-bipyridin-5-yl)-7-methy...)Show SMILES CN1C2CCC1C(C2)c1cncc(c1)-c1ccc(F)nc1 |TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-3-4-16(21)15(7-14)13-6-12(8-19-9-13)11-2-5-17(18)20-10-11/h2,5-6,8-10,14-16H,3-4,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50263171

((+)-2-(6'-[18F]fluoro-3,3'-bipyridin-5-yl)-7-methy...)Show SMILES CN1C2CCC1C(C2)c1cncc(c1)-c1ccc(F)nc1 |TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-3-4-16(21)15(7-14)13-6-12(8-19-9-13)11-2-5-17(18)20-10-11/h2,5-6,8-10,14-16H,3-4,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
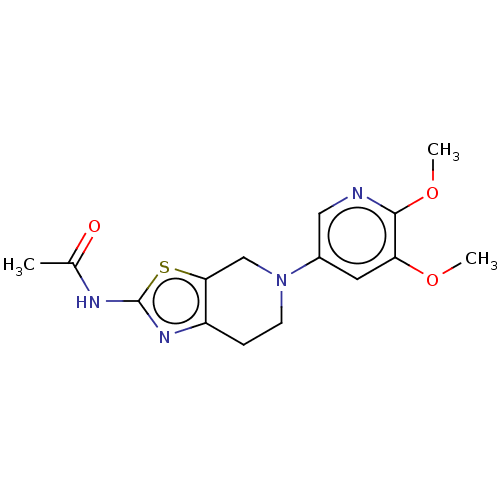
(Homo sapiens (Human)) | BDBM50093437
(CHEMBL3586668)Show InChI InChI=1S/C20H32O2/c1-19-7-5-14(21)10-13(19)3-4-15-16(19)6-8-20(2)17(15)9-12-11-22-18(12)20/h12-18,21H,3-11H2,1-2H3/t12-,13+,14-,15-,16+,17+,18+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50263095
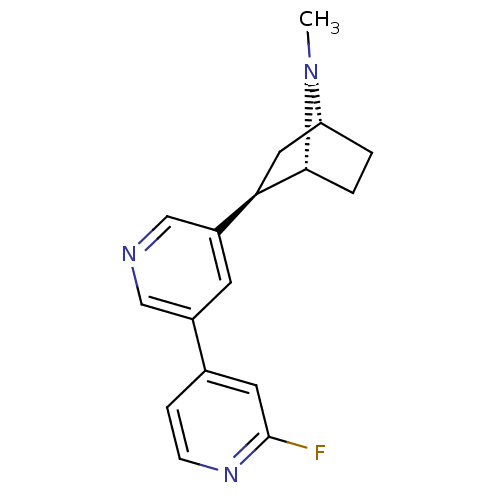
((+)-7-Methyl-2-exo-[3'-(2-fluoropyridin-4-yl)-5'-p...)Show SMILES CN1[C@@H]2CC[C@@H]1[C@H](C2)c1cncc(c1)-c1ccnc(F)c1 |r,TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-2-3-16(21)15(8-14)13-6-12(9-19-10-13)11-4-5-20-17(18)7-11/h4-7,9-10,14-16H,2-3,8H2,1H3/t14-,15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50263095

((+)-7-Methyl-2-exo-[3'-(2-fluoropyridin-4-yl)-5'-p...)Show SMILES CN1[C@@H]2CC[C@@H]1[C@H](C2)c1cncc(c1)-c1ccnc(F)c1 |r,TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-2-3-16(21)15(8-14)13-6-12(9-19-10-13)11-4-5-20-17(18)7-11/h4-7,9-10,14-16H,2-3,8H2,1H3/t14-,15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093399
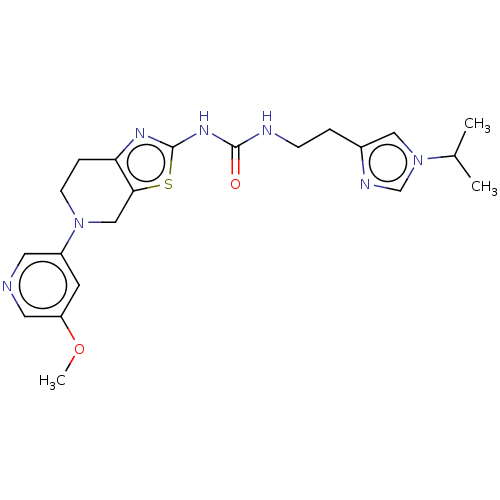
(CHEMBL3586673)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(cn3)C(C)C)sc2C1 Show InChI InChI=1S/C21H27N7O2S/c1-14(2)28-11-15(24-13-28)4-6-23-20(29)26-21-25-18-5-7-27(12-19(18)31-21)16-8-17(30-3)10-22-9-16/h8-11,13-14H,4-7,12H2,1-3H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093434
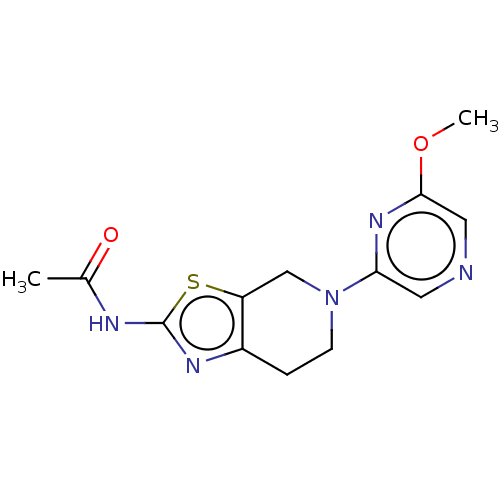
(CHEMBL3586670)Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50263095

((+)-7-Methyl-2-exo-[3'-(2-fluoropyridin-4-yl)-5'-p...)Show SMILES CN1[C@@H]2CC[C@@H]1[C@H](C2)c1cncc(c1)-c1ccnc(F)c1 |r,TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-2-3-16(21)15(8-14)13-6-12(9-19-10-13)11-4-5-20-17(18)7-11/h4-7,9-10,14-16H,2-3,8H2,1H3/t14-,15-,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50263095

((+)-7-Methyl-2-exo-[3'-(2-fluoropyridin-4-yl)-5'-p...)Show SMILES CN1[C@@H]2CC[C@@H]1[C@H](C2)c1cncc(c1)-c1ccnc(F)c1 |r,TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-14-2-3-16(21)15(8-14)13-6-12(9-19-10-13)11-4-5-20-17(18)7-11/h4-7,9-10,14-16H,2-3,8H2,1H3/t14-,15-,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373939
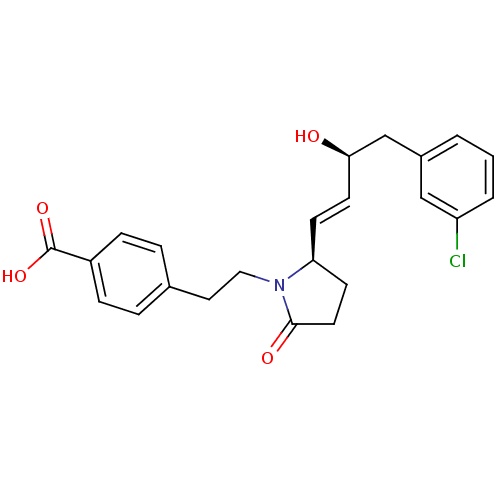
(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030756
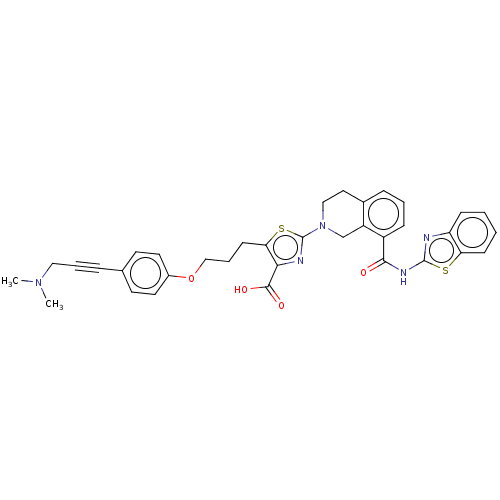
(CHEMBL3342197)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C35H33N5O4S2/c1-39(2)19-6-8-23-14-16-25(17-15-23)44-21-7-13-30-31(33(42)43)37-35(46-30)40-20-18-24-9-5-10-26(27(24)22-40)32(41)38-34-36-28-11-3-4-12-29(28)45-34/h3-5,9-12,14-17H,7,13,18-22H2,1-2H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha-2-beta-2 nACHR expressed in human HEK293 cells |
J Med Chem 49: 2673-6 (2006)
Article DOI: 10.1021/jm051196m
BindingDB Entry DOI: 10.7270/Q2GX4F96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-6
(Rattus norvegicus) | BDBM50263130
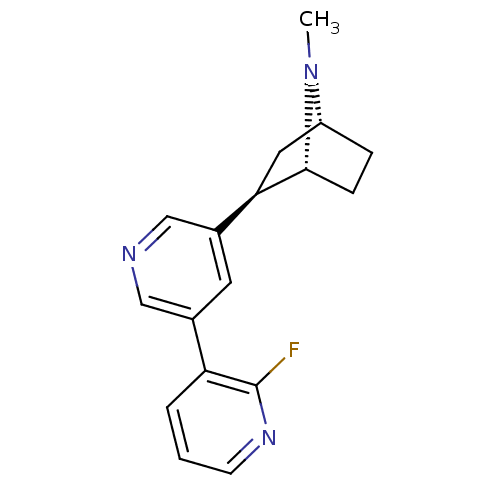
((+)-7-Methyl-2-exo-[3'-(2-fluoropyridin-3-yl)-5'-p...)Show SMILES CN1[C@@H]2CC[C@@H]1[C@H](C2)c1cncc(c1)-c1cccnc1F |r,TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-13-4-5-16(21)15(8-13)12-7-11(9-19-10-12)14-3-2-6-20-17(14)18/h2-3,6-7,9-10,13,15-16H,4-5,8H2,1H3/t13-,15-,16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha6beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Rattus norvegicus) | BDBM50263130

((+)-7-Methyl-2-exo-[3'-(2-fluoropyridin-3-yl)-5'-p...)Show SMILES CN1[C@@H]2CC[C@@H]1[C@H](C2)c1cncc(c1)-c1cccnc1F |r,TLB:8:6:3.4:1| Show InChI InChI=1S/C17H18FN3/c1-21-13-4-5-16(21)15(8-13)12-7-11(9-19-10-12)14-3-2-6-20-17(14)18/h2-3,6-7,9-10,13,15-16H,4-5,8H2,1H3/t13-,15-,16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha6beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50295954
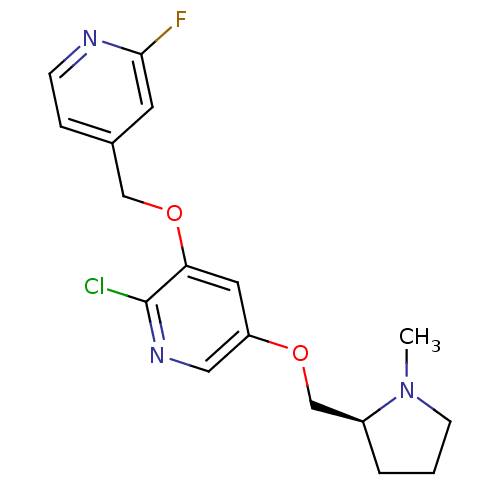
(2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...)Show SMILES CN1CCC[C@H]1COc1cnc(Cl)c(OCc2ccnc(F)c2)c1 |r| Show InChI InChI=1S/C17H19ClFN3O2/c1-22-6-2-3-13(22)11-23-14-8-15(17(18)21-9-14)24-10-12-4-5-20-16(19)7-12/h4-5,7-9,13H,2-3,6,10-11H2,1H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50295954

(2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...)Show SMILES CN1CCC[C@H]1COc1cnc(Cl)c(OCc2ccnc(F)c2)c1 |r| Show InChI InChI=1S/C17H19ClFN3O2/c1-22-6-2-3-13(22)11-23-14-8-15(17(18)21-9-14)24-10-12-4-5-20-16(19)7-12/h4-5,7-9,13H,2-3,6,10-11H2,1H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148931
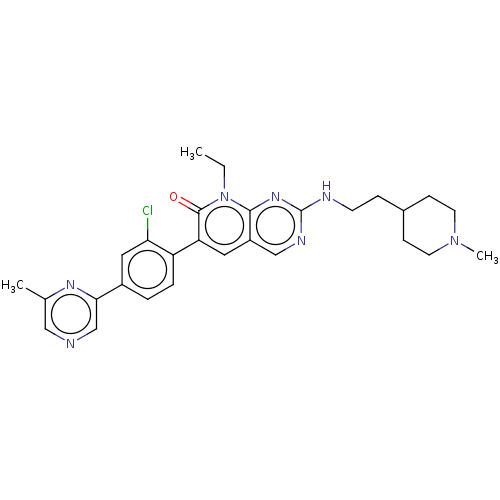
(CHEMBL3770186)Show SMILES CCn1c2nc(NCCC3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncc(C)n2)c1=O Show InChI InChI=1S/C28H32ClN7O/c1-4-36-26-21(16-32-28(34-26)31-10-7-19-8-11-35(3)12-9-19)13-23(27(36)37)22-6-5-20(14-24(22)29)25-17-30-15-18(2)33-25/h5-6,13-17,19H,4,7-12H2,1-3H3,(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112517
BindingDB Entry DOI: 10.7270/Q2Q243W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093436
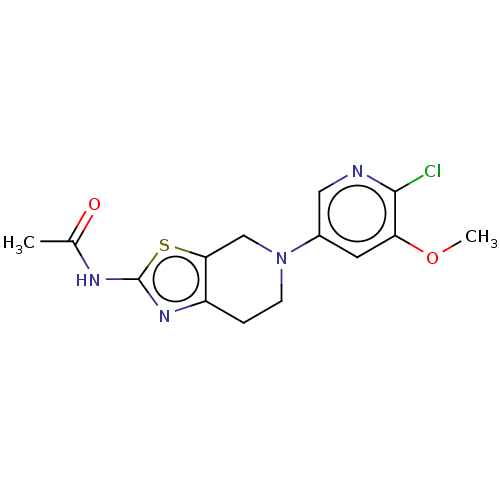
(CHEMBL3586669)Show InChI InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/p-2/b7-3+,8-4+/t19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Nicotinic acetylcholine receptor alpha2-beta2 |
J Med Chem 46: 921-4 (2003)
Article DOI: 10.1021/jm025613w
BindingDB Entry DOI: 10.7270/Q2P271VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine |
Bioorg Med Chem Lett 15: 4385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.039
BindingDB Entry DOI: 10.7270/Q2H41V62 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards rat forebrain nicotinic acetylcholine receptor using [3H]EB as radioligand |
Bioorg Med Chem Lett 14: 1845-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.105
BindingDB Entry DOI: 10.7270/Q2TB17G2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine |
Bioorg Med Chem Lett 14: 1855-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.10.071
BindingDB Entry DOI: 10.7270/Q269753N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 98-107 (2004)
Article DOI: 10.1124/jpet.104.066787
BindingDB Entry DOI: 10.7270/Q2XG9PQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins UniVersity School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nAChR expressed in HEK293 cells |
J Med Chem 51: 4751-64 (2008)
Article DOI: 10.1021/jm800323d
BindingDB Entry DOI: 10.7270/Q23N249J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data