 Found 12 hits with Last Name = 'baculi' and Initial = 'f'
Found 12 hits with Last Name = 'baculi' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 92 kDa gelatinase MMP-9 at 100 uM |
Bioorg Med Chem Lett 5: 1637-1642 (1995)
Article DOI: 10.1016/0960-894X(95)00282-X
BindingDB Entry DOI: 10.7270/Q2J38SJB |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant interstitial collagenase MMP-1 at 100 uM |
Bioorg Med Chem Lett 5: 1637-1642 (1995)
Article DOI: 10.1016/0960-894X(95)00282-X
BindingDB Entry DOI: 10.7270/Q2J38SJB |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50545275

(CHEMBL4645677)Show SMILES Fc1ccc(N2CCN(Cc3ccc(COc4cccc5C(=O)N(Cc45)C4CCC(=O)NC4=O)cc3)CC2)c(F)c1 Show InChI InChI=1S/C31H30F2N4O4/c32-22-8-9-26(25(33)16-22)36-14-12-35(13-15-36)17-20-4-6-21(7-5-20)19-41-28-3-1-2-23-24(28)18-37(31(23)40)27-10-11-29(38)34-30(27)39/h1-9,16,27H,10-15,17-19H2,(H,34,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of adrenergic receptor alpha1 (unknown origin) |
J Med Chem 63: 6648-6676 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01928
BindingDB Entry DOI: 10.7270/Q2KS6W4N |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-3 (Stromelysin) at 100 uM |
Bioorg Med Chem Lett 5: 1637-1642 (1995)
Article DOI: 10.1016/0960-894X(95)00282-X
BindingDB Entry DOI: 10.7270/Q2J38SJB |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM65497
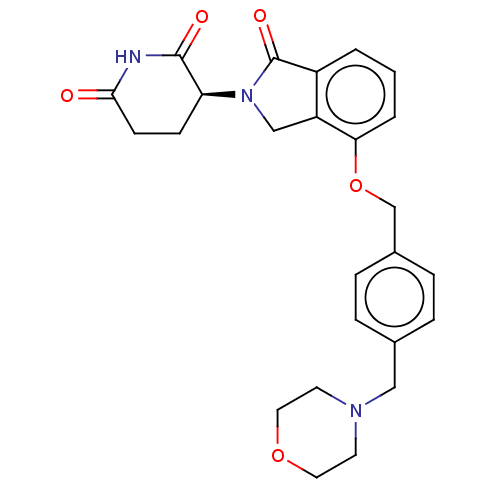
(CC-220 (Compound 6) | US9694015, 6.4S)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@H]1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Celgene Corporation
| Assay Description
The 6XHis-tagged full length human CRBN bound to full length human DDB1 used in the assay was purified as described elsewhere with the exception that... |
J Med Chem 61: 535-542 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01921
BindingDB Entry DOI: 10.7270/Q2PC30HG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50284909
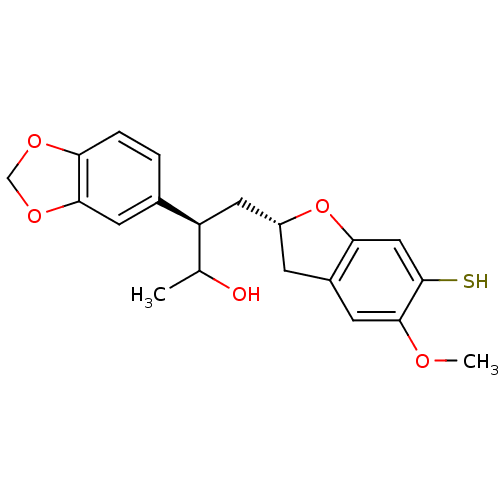
((R)-3-Benzo[1,3]dioxol-5-yl-4-((S)-6-mercapto-5-me...)Show SMILES COc1cc2C[C@H](C[C@@H](C(C)O)c3ccc4OCOc4c3)Oc2cc1S Show InChI InChI=1S/C20H22O5S/c1-11(21)15(12-3-4-16-18(6-12)24-10-23-16)8-14-5-13-7-19(22-2)20(26)9-17(13)25-14/h3-4,6-7,9,11,14-15,21,26H,5,8,10H2,1-2H3/t11?,14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-3 (Stromelysin) at 10 uM |
Bioorg Med Chem Lett 5: 1637-1642 (1995)
Article DOI: 10.1016/0960-894X(95)00282-X
BindingDB Entry DOI: 10.7270/Q2J38SJB |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50545276
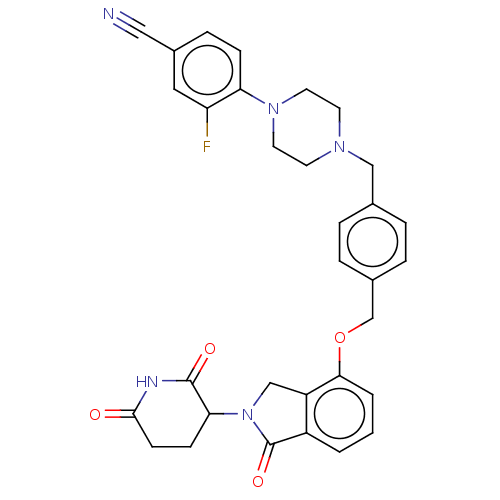
(CHEMBL4637801)Show SMILES Fc1cc(ccc1N1CCN(Cc2ccc(COc3cccc4C(=O)N(Cc34)C3CCC(=O)NC3=O)cc2)CC1)C#N Show InChI InChI=1S/C32H30FN5O4/c33-26-16-23(17-34)8-9-27(26)37-14-12-36(13-15-37)18-21-4-6-22(7-5-21)20-42-29-3-1-2-24-25(29)19-38(32(24)41)28-10-11-30(39)35-31(28)40/h1-9,16,28H,10-15,18-20H2,(H,35,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of adrenergic receptor alpha1 (unknown origin) |
J Med Chem 63: 6648-6676 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01928
BindingDB Entry DOI: 10.7270/Q2KS6W4N |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213210
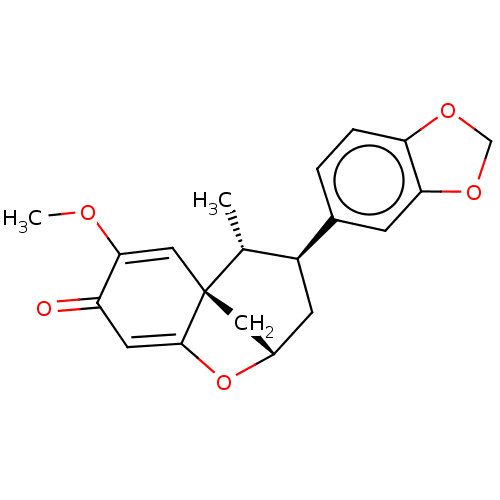
(FUTOENONE)Show SMILES [H][C@]12C[C@@]3(C=C(OC)C(=O)C=C3O1)[C@H](C)[C@H](C2)c1ccc2OCOc2c1 |c:10,t:4| Show InChI InChI=1S/C20H20O5/c1-11-14(12-3-4-16-17(5-12)24-10-23-16)6-13-8-20(11)9-18(22-2)15(21)7-19(20)25-13/h3-5,7,9,11,13-14H,6,8,10H2,1-2H3/t11-,13+,14+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease-3 (Stromelysin) at 10 uM |
Bioorg Med Chem Lett 5: 1637-1642 (1995)
Article DOI: 10.1016/0960-894X(95)00282-X
BindingDB Entry DOI: 10.7270/Q2J38SJB |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM65456
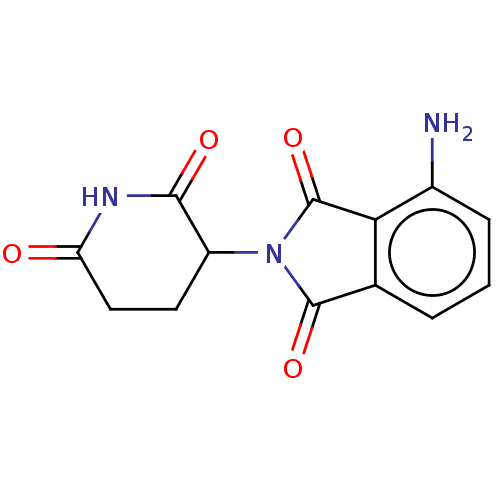
(19171-19-8 | 4-amino-2-(2,6-dioxopiperidin-3-yl)is...)Show InChI InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Celgene Corporation
| Assay Description
The 6XHis-tagged full length human CRBN bound to full length human DDB1 used in the assay was purified as described elsewhere with the exception that... |
J Med Chem 61: 535-542 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01921
BindingDB Entry DOI: 10.7270/Q2PC30HG |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM65454
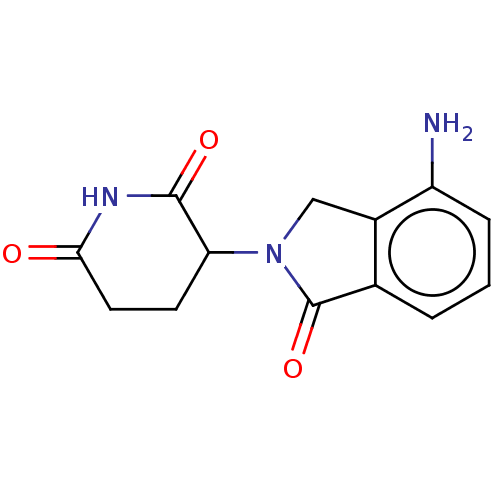
(191732-72-6 | CC-5013 | Lenalidomide | Revimid | R...)Show InChI InChI=1S/C13H13N3O3/c14-9-3-1-2-7-8(9)6-16(13(7)19)10-4-5-11(17)15-12(10)18/h1-3,10H,4-6,14H2,(H,15,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Celgene Corporation
| Assay Description
The 6XHis-tagged full length human CRBN bound to full length human DDB1 used in the assay was purified as described elsewhere with the exception that... |
J Med Chem 61: 535-542 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01921
BindingDB Entry DOI: 10.7270/Q2PC30HG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50545275

(CHEMBL4645677)Show SMILES Fc1ccc(N2CCN(Cc3ccc(COc4cccc5C(=O)N(Cc45)C4CCC(=O)NC4=O)cc3)CC2)c(F)c1 Show InChI InChI=1S/C31H30F2N4O4/c32-22-8-9-26(25(33)16-22)36-14-12-35(13-15-36)17-20-4-6-21(7-5-20)19-41-28-3-1-2-23-24(28)18-37(31(23)40)27-10-11-29(38)34-30(27)39/h1-9,16,27H,10-15,17-19H2,(H,34,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at D2S (unknown origin) |
J Med Chem 63: 6648-6676 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01928
BindingDB Entry DOI: 10.7270/Q2KS6W4N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50545276

(CHEMBL4637801)Show SMILES Fc1cc(ccc1N1CCN(Cc2ccc(COc3cccc4C(=O)N(Cc34)C3CCC(=O)NC3=O)cc2)CC1)C#N Show InChI InChI=1S/C32H30FN5O4/c33-26-16-23(17-34)8-9-27(26)37-14-12-36(13-15-37)18-21-4-6-22(7-5-21)20-42-29-3-1-2-24-25(29)19-38(32(24)41)28-10-11-30(39)35-31(28)40/h1-9,16,28H,10-15,18-20H2,(H,35,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at D2S (unknown origin) |
J Med Chem 63: 6648-6676 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01928
BindingDB Entry DOI: 10.7270/Q2KS6W4N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data