

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86298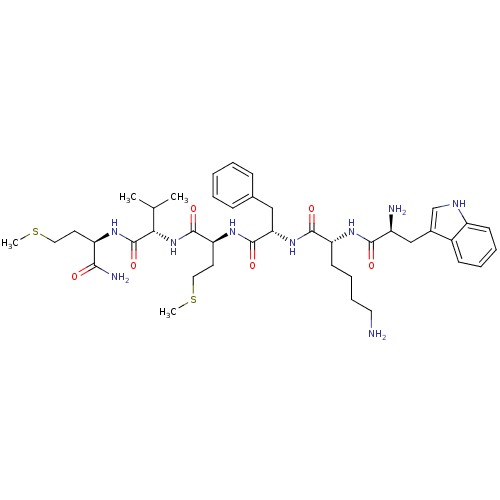 (WKFMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86301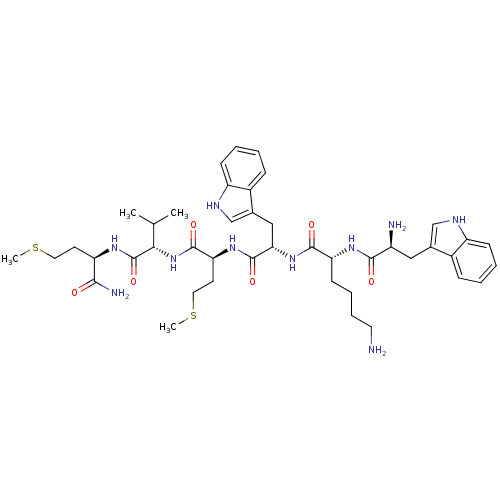 (WKWMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86299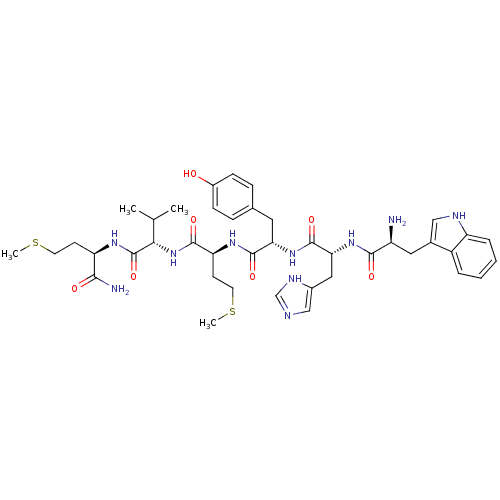 (WHYMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86297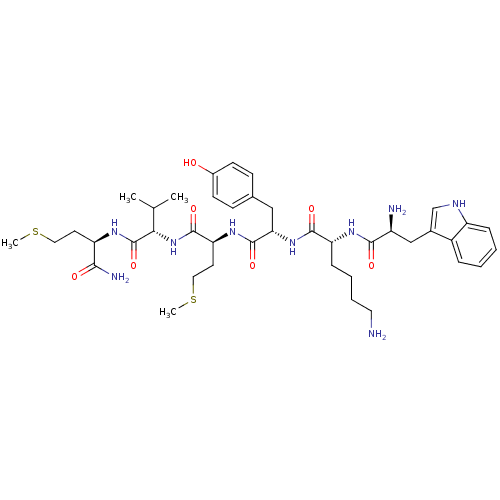 (WKYMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430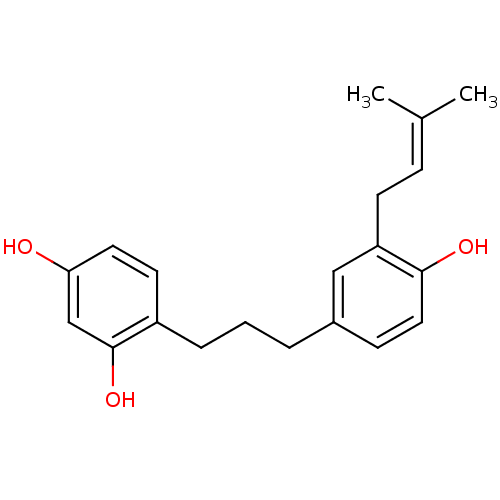 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 48.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86308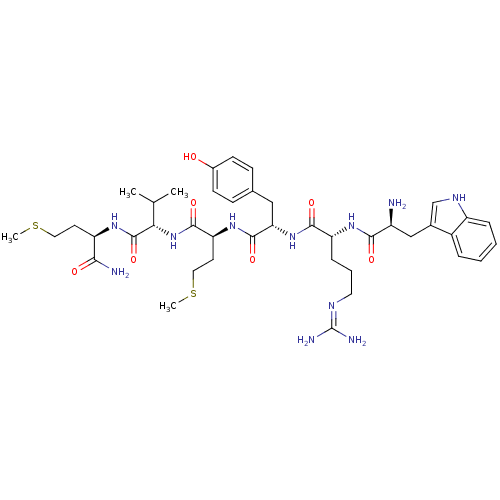 (WRYMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86300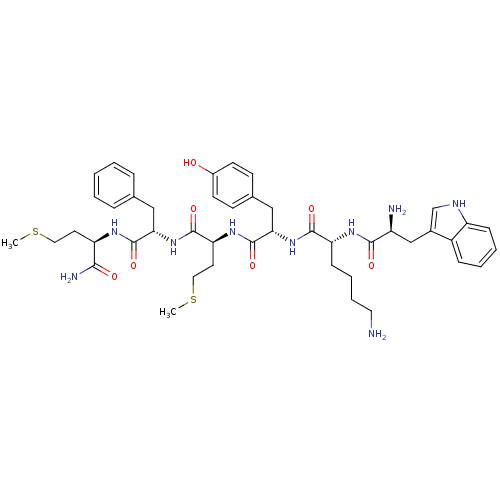 (WKYM(F/W)m-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86302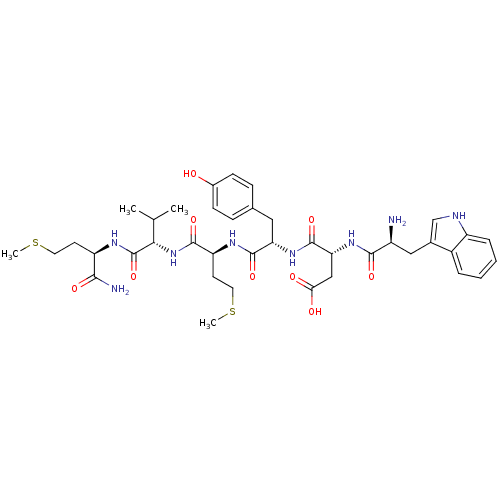 (WDYMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001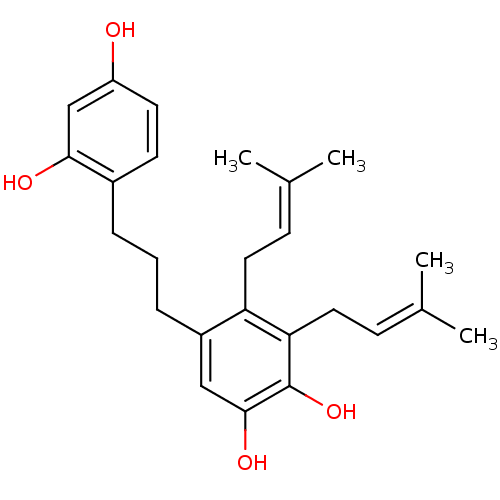 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86303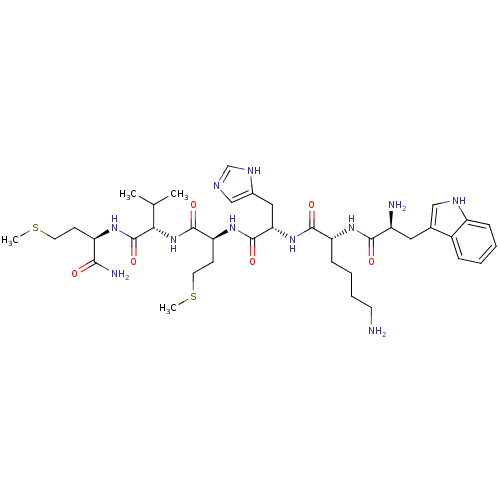 (WKHMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86306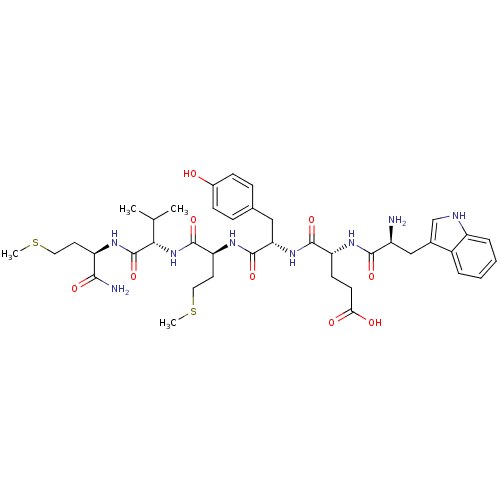 (WEYMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190499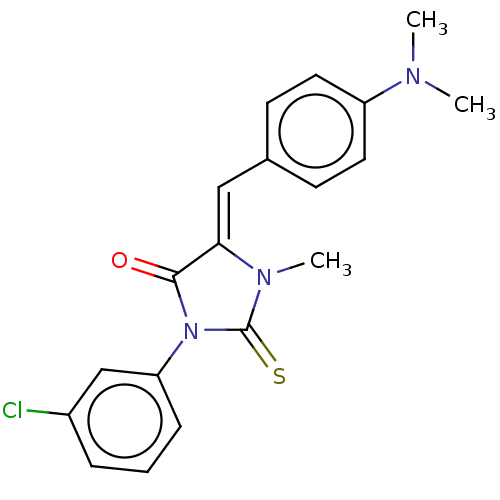 (CHEMBL3827985) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86305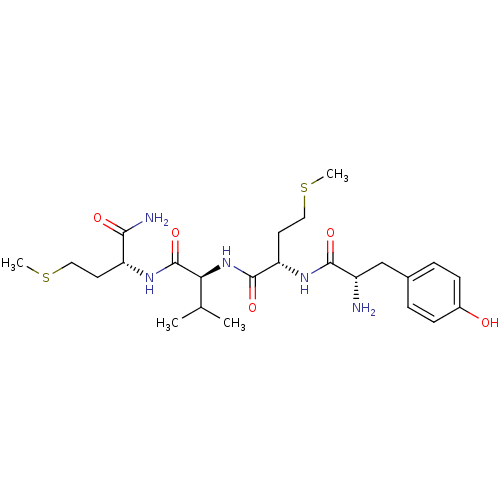 (YMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 567 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86307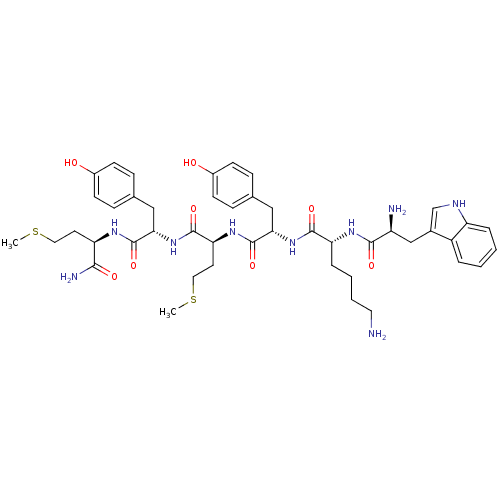 (WKYMYm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 671 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190499 (CHEMBL3827985) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190343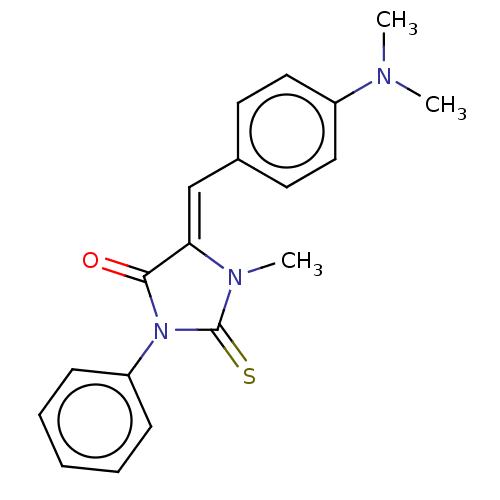 (CHEMBL3827795) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190331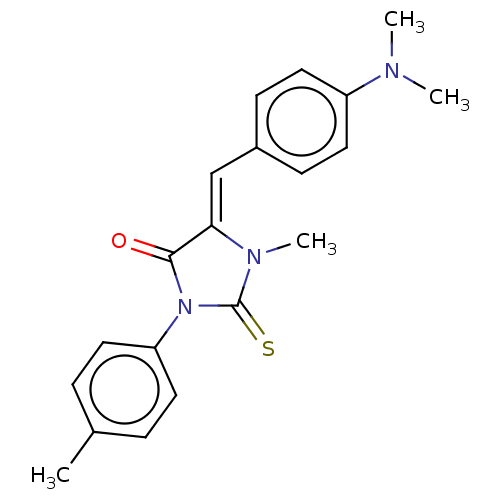 (CHEMBL3828176) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86304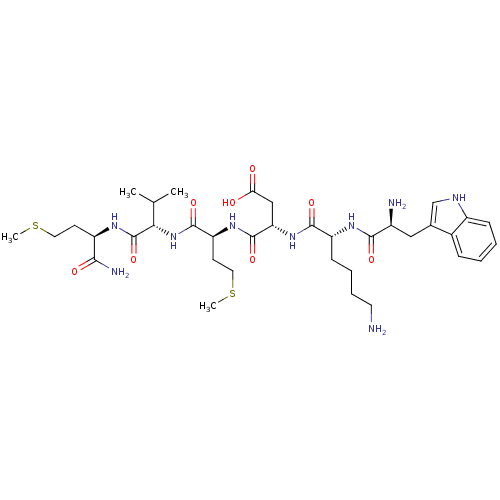 (WKDMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM86296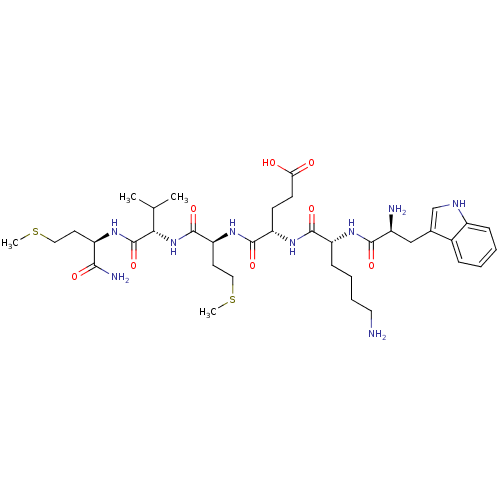 (WKEMVm-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by PDSP Ki Database | Mol Pharmacol 64: 841-7 (2003) Article DOI: 10.1124/mol.64.4.841 BindingDB Entry DOI: 10.7270/Q2WQ02D4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190390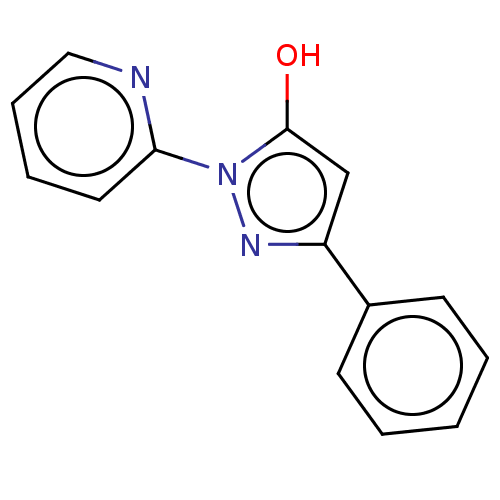 (CHEMBL3828444) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190343 (CHEMBL3827795) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190331 (CHEMBL3828176) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190497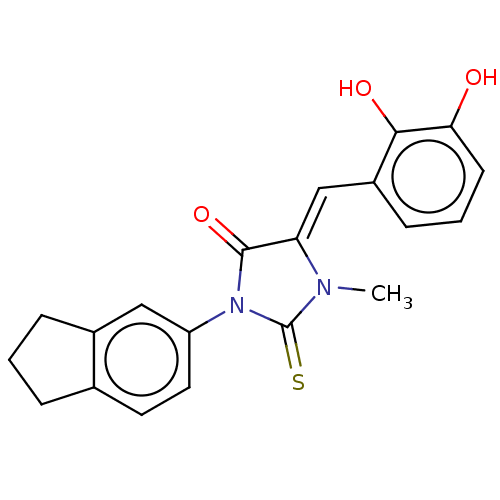 (CHEMBL3827227) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190330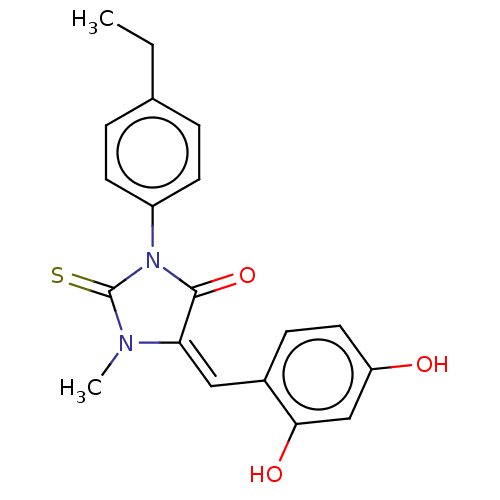 (CHEMBL3828737) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190328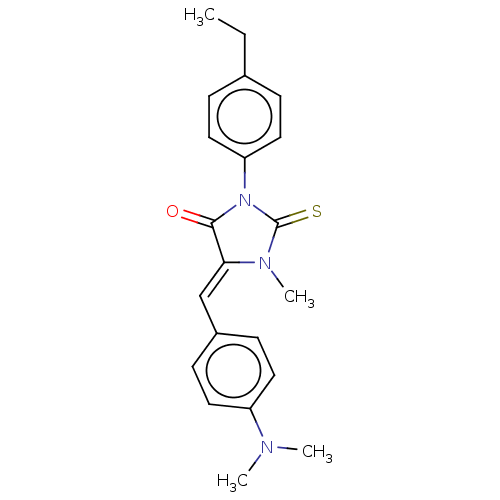 (CHEMBL3827342) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190390 (CHEMBL3828444) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol-trisphosphate 3-kinase A (Homo sapiens (Human)) | BDBM82310 (Purine, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | -31.9 | 1.02E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
New York University | Assay Description IP3K reactions were carried out in a 100ul solution that contained Tris-Cl, EGTA, ATP, DTT, 2,3-diphosphoglycerate, D-l(1,4,5)P3, [3H]-l(1,4,5)P3 and... | Chembiochem 3: 897-901 (2002) Article DOI: 10.1002/1439-7633(20020902)3:9 BindingDB Entry DOI: 10.7270/Q2DJ5D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190328 (CHEMBL3827342) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190347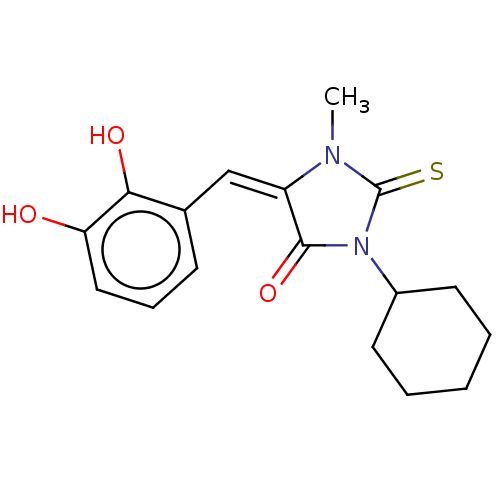 (CHEMBL3827481) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190498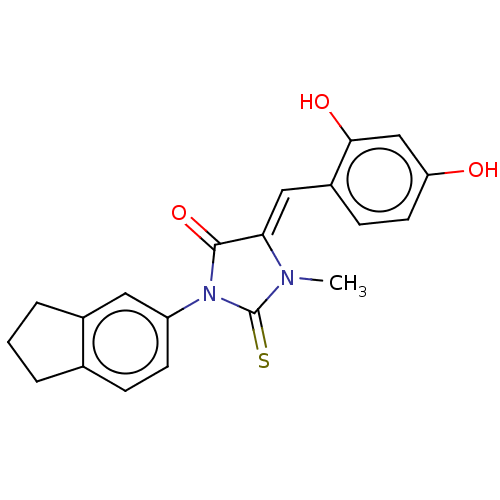 (CHEMBL3827096) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190497 (CHEMBL3827227) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190498 (CHEMBL3827096) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190347 (CHEMBL3827481) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190503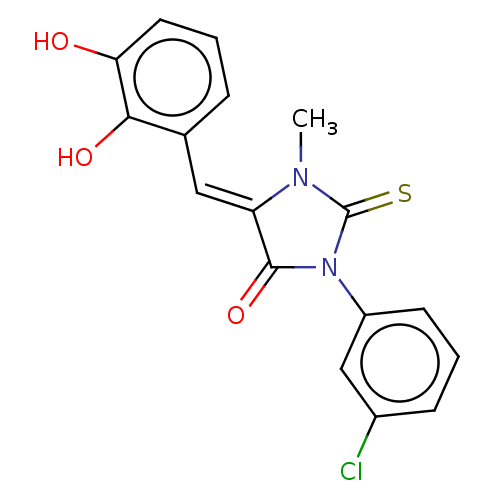 (CHEMBL3827569) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190391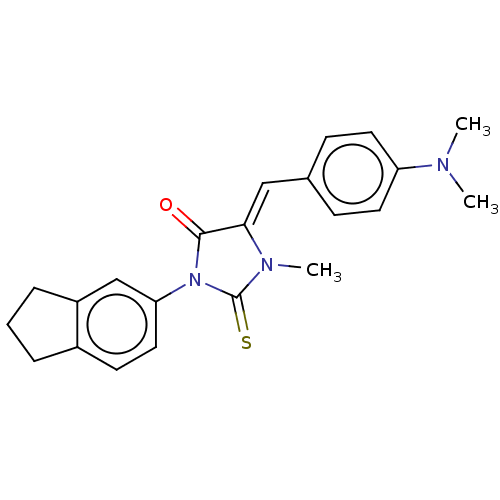 (CHEMBL3826995) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 8.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190330 (CHEMBL3828737) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190503 (CHEMBL3827569) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254429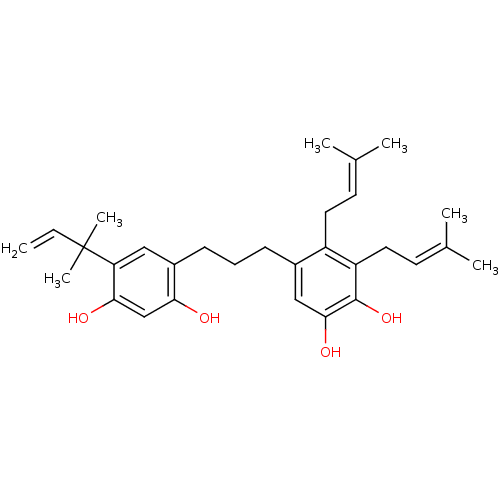 (CHEMBL466200 | kazinol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254429 (CHEMBL466200 | kazinol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190346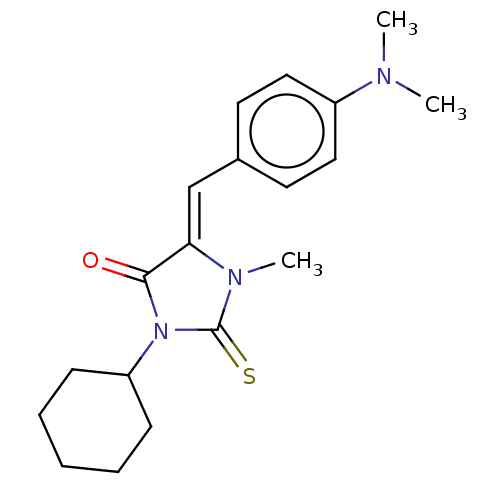 (CHEMBL3827497) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190391 (CHEMBL3826995) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 4 (Homo sapiens (Human)) | BDBM50190344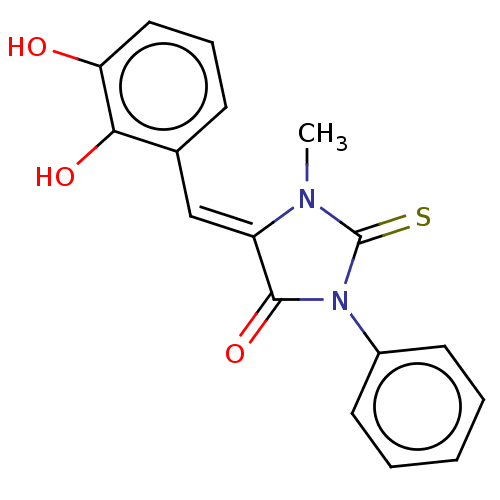 (CHEMBL3828498) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX4 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase 1 (Homo sapiens (Human)) | BDBM50190348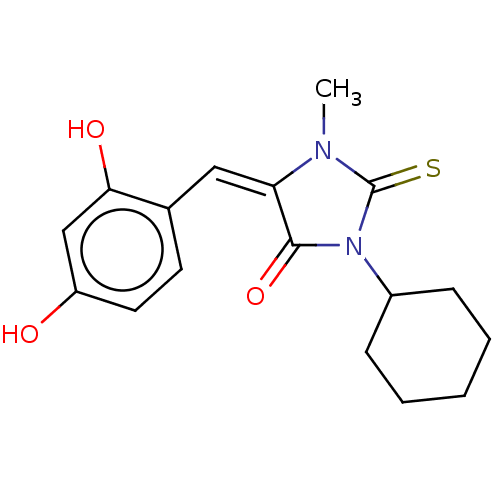 (CHEMBL3827421) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human NOX1 expressed in Drosophila DUOX knockdown model assessed as decrease in ROS production by lucigenin chemiluminescence assay | Bioorg Med Chem 24: 4144-4151 (2016) Article DOI: 10.1016/j.bmc.2016.06.056 BindingDB Entry DOI: 10.7270/Q2XK8HHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 146 total ) | Next | Last >> |