

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545382 (CHEMBL4646450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545379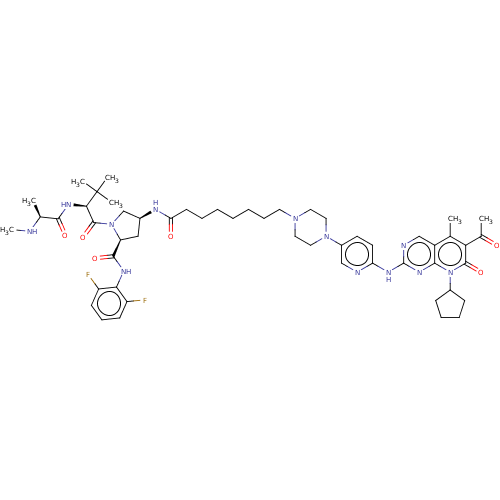 (CHEMBL4645010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545380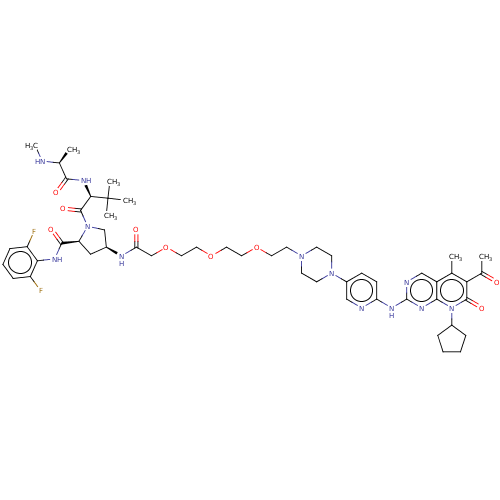 (CHEMBL4645458) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545383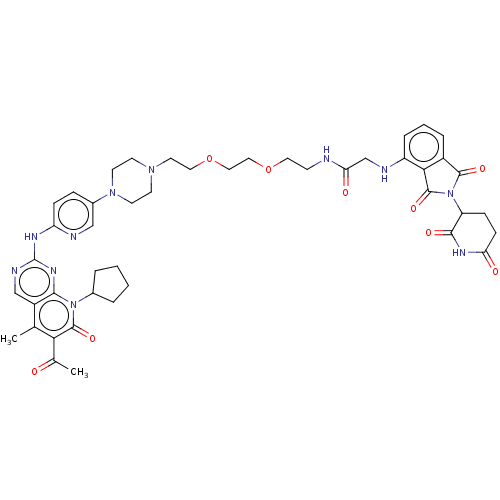 (CHEMBL4636355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545382 (CHEMBL4646450) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM6309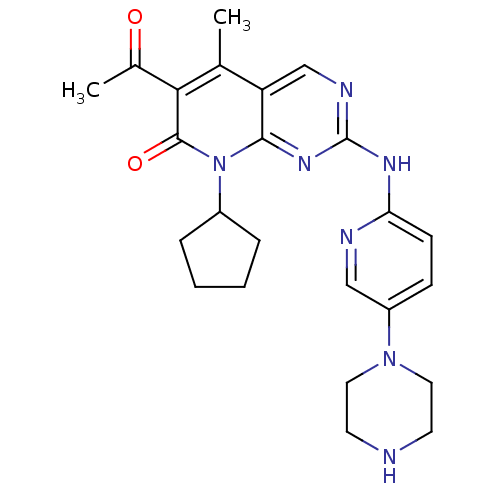 (6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545379 (CHEMBL4645010) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase (Staphylococcus aureus) | BDBM50444582 (CHEMBL3099715) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal 6xHis-tagged full length recombinant Staphylococcus aureus DNA ligase (1 to 312) expressed in Escherichia coli BL21 (DE3) us... | ACS Med Chem Lett 4: 1208-12 (2013) Article DOI: 10.1021/ml4003277 BindingDB Entry DOI: 10.7270/Q2J967V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545381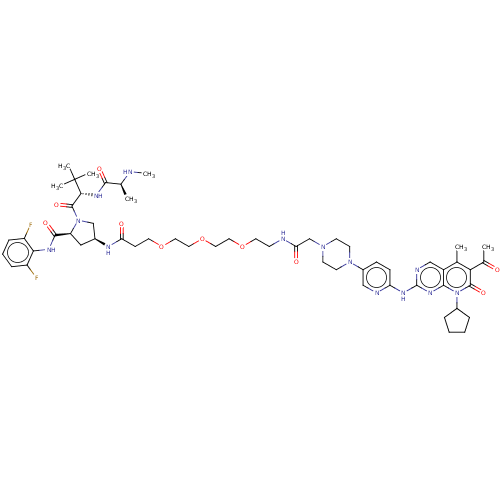 (CHEMBL4649032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G1/S-specific cyclin-D2 (Homo sapiens (Human)) | BDBM6309 (6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D2 (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545380 (CHEMBL4645458) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545383 (CHEMBL4636355) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545384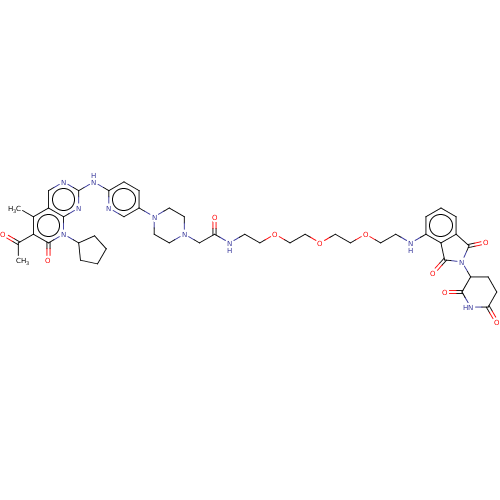 (CHEMBL4548417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545376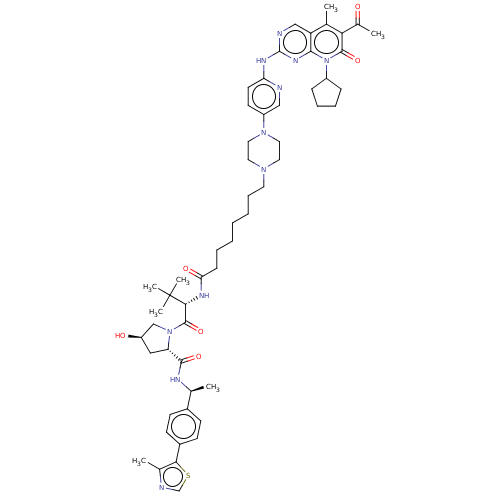 (CHEMBL4638166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM6309 (6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545378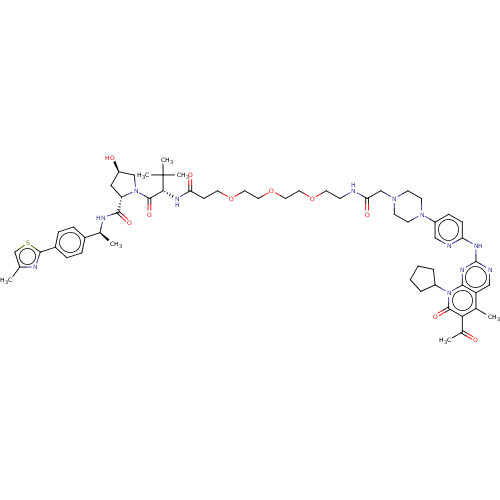 (CHEMBL4638298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545381 (CHEMBL4649032) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM50545377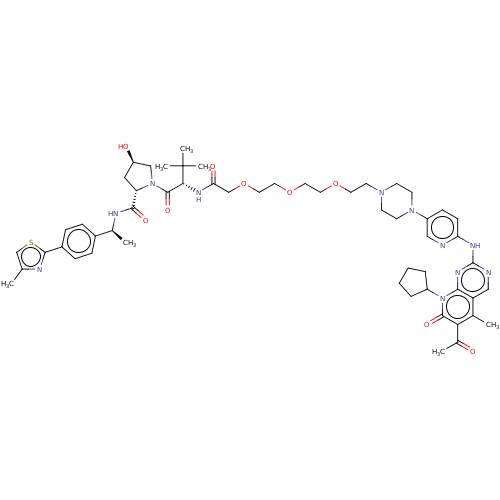 (CHEMBL4640158) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclin-D1 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545384 (CHEMBL4548417) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM621246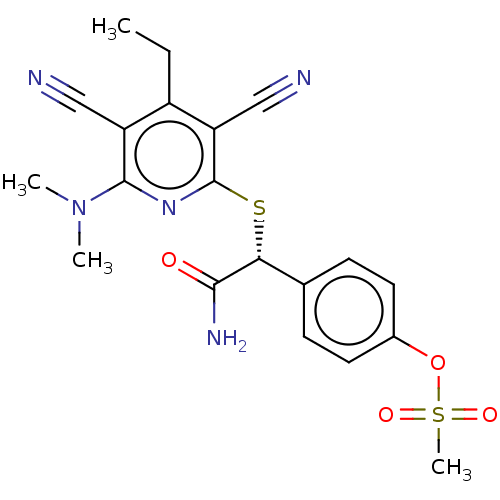 (US11771711, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 [601-1600] (Homo sapiens (Human)) | BDBM491448 (US10975056, Example 422 | US11771711, Reference co...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491120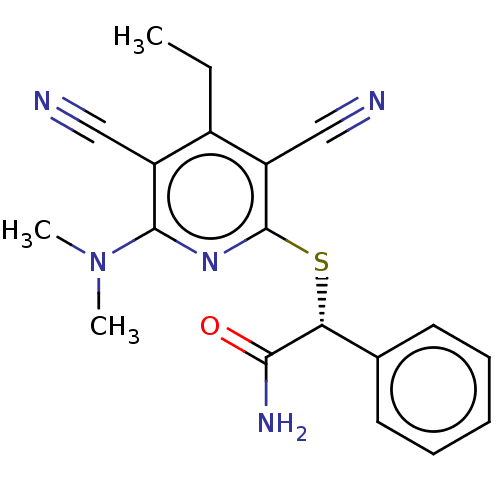 ((R)-2-((3,5-dicyano-6-(dimethylamino)-4-ethylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM6309 (6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM621248 (4-(2-amino-1-((3,5-dicyano-6-(dimethylamino)-4-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545378 (CHEMBL4638298) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491224 (US10975056, Example 168 | US11771711, Reference co...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545377 (CHEMBL4640158) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545376 (CHEMBL4638166) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50545376 (CHEMBL4638166) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin-D3 using RB protein as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127106 BindingDB Entry DOI: 10.7270/Q2JW8JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase (Staphylococcus aureus) | BDBM50444581 (CHEMBL3099713) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal 6xHis-tagged full length recombinant Staphylococcus aureus DNA ligase (1 to 312) expressed in Escherichia coli BL21 (DE3) us... | ACS Med Chem Lett 4: 1208-12 (2013) Article DOI: 10.1021/ml4003277 BindingDB Entry DOI: 10.7270/Q2J967V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase (Staphylococcus aureus) | BDBM50444580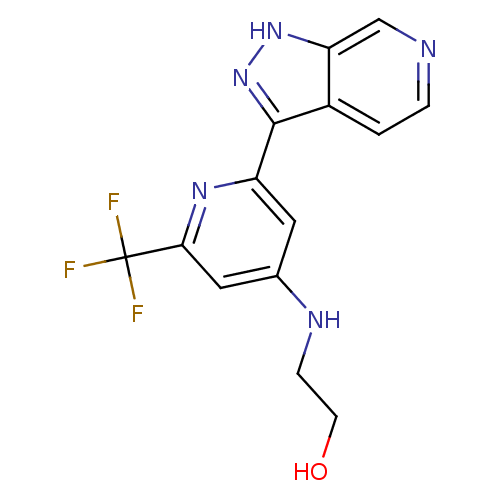 (CHEMBL3099714) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal 6xHis-tagged full length recombinant Staphylococcus aureus DNA ligase (1 to 312) expressed in Escherichia coli BL21 (DE3) us... | ACS Med Chem Lett 4: 1208-12 (2013) Article DOI: 10.1021/ml4003277 BindingDB Entry DOI: 10.7270/Q2J967V2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491060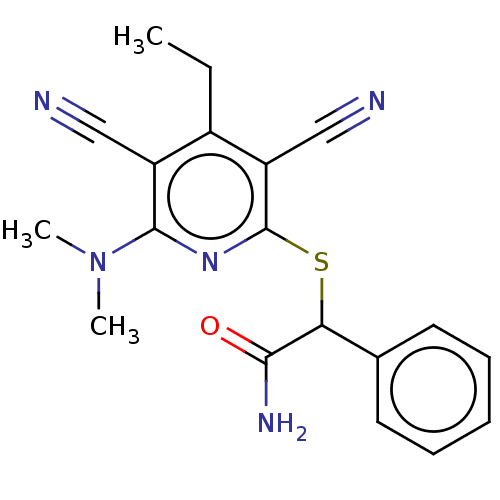 (BDBM491430 | US10975056, Example 3 | US10975056, E...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491061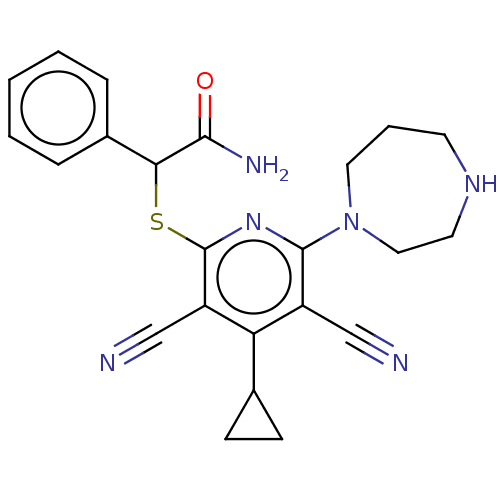 (US10975056, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491062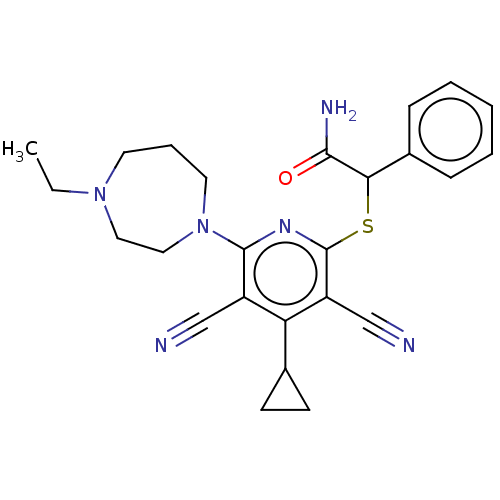 (2-{[3,5-dicyano-4-cyclopropyl-6-(4-ethyl-1,4-diaze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491063 (US10975056, Example 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491064 (2-{[3,5-dicyano-4-ethyl-6-(4-ethyl-1,4-diazepan-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491071 (US10975056, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491073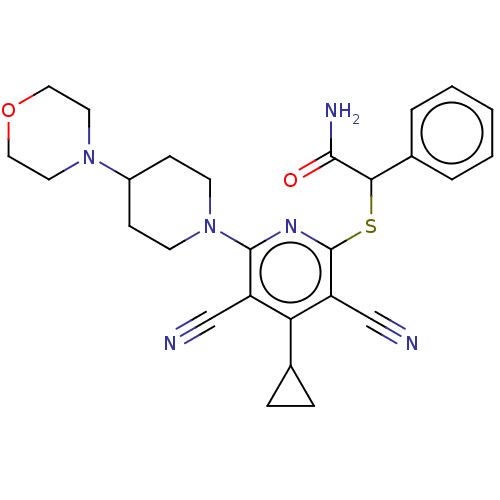 (US10975056, Example 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491074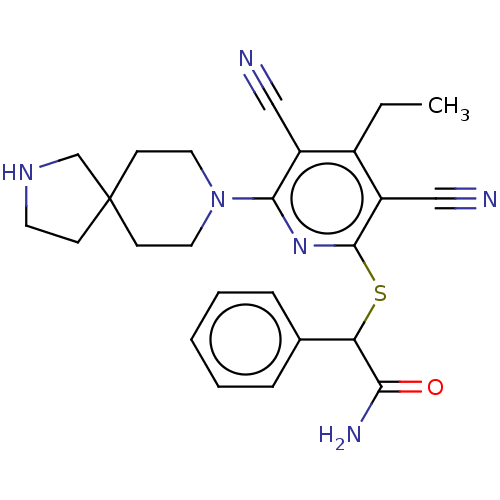 (2-((3,5-dicyano-4-ethyl-6-(2,8-diazaspiro[4.5]deca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491080 (US10975056, Example 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491081 (US10975056, Example 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491082 (US10975056, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491075 (US10975056, Example 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491077 (US10975056, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491084 (US10975056, Example 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491204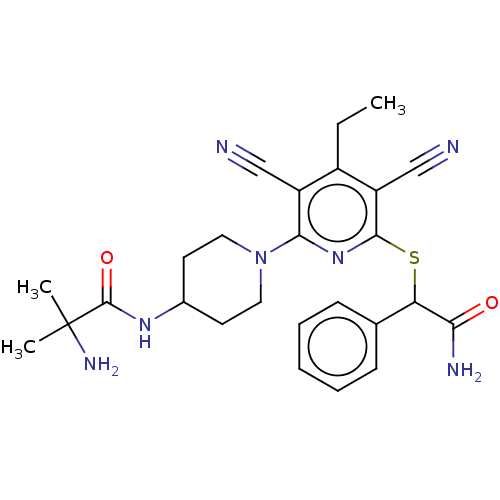 (US10975056, Example 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491205 (US10975056, Example 149) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491209 (US10975056, Example 153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491212 (US10975056, Example 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491223 (US10975056, Example 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 703 total ) | Next | Last >> |