

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100263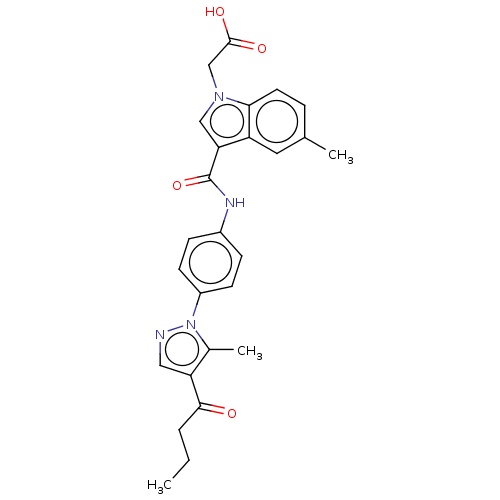 (CHEMBL3326907) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536438 (CHEMBL4534488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536438 (CHEMBL4534488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536429 (CHEMBL4581491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425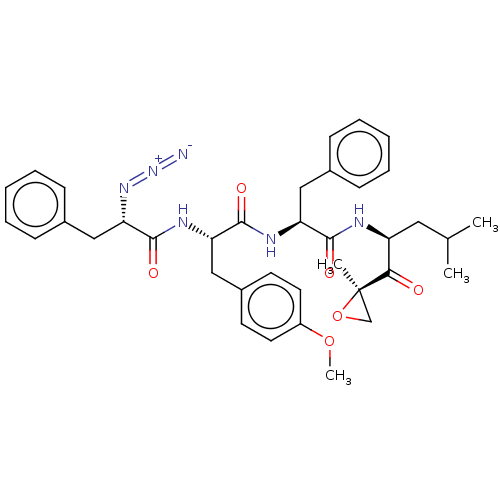 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536429 (CHEMBL4581491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536442 (CHEMBL4570216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536439 (CHEMBL4581001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100269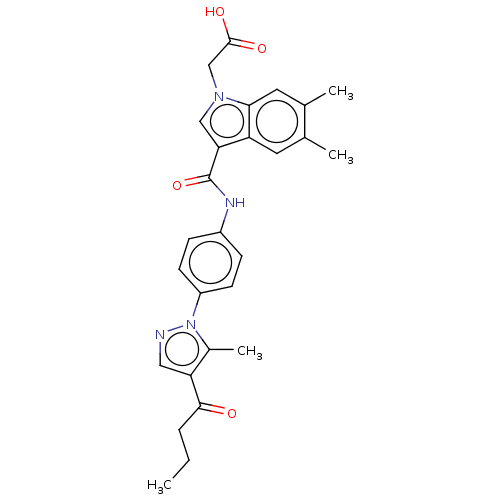 (CHEMBL3325627) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100262 (CHEMBL3326906) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100245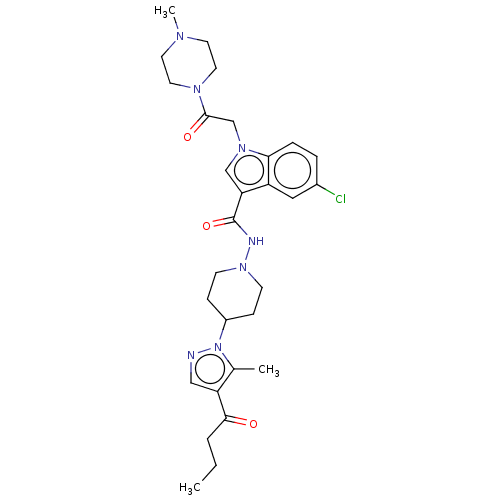 (CHEMBL3325894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100195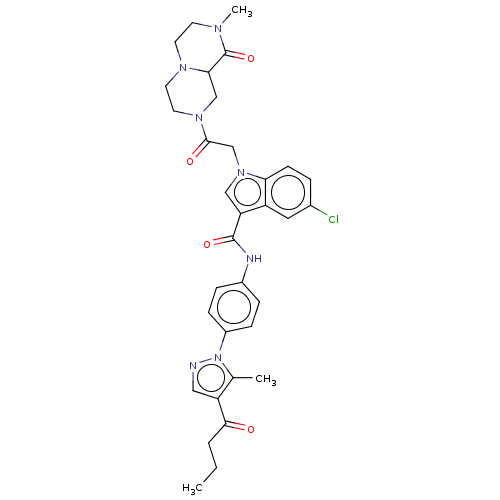 (CHEMBL3325805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100195 (CHEMBL3325805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100195 (CHEMBL3325805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536448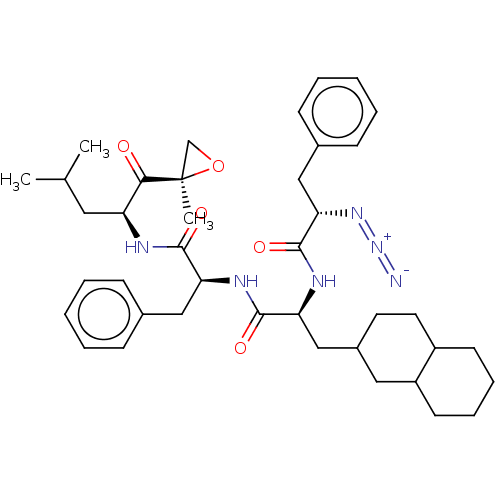 (CHEMBL4532975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536457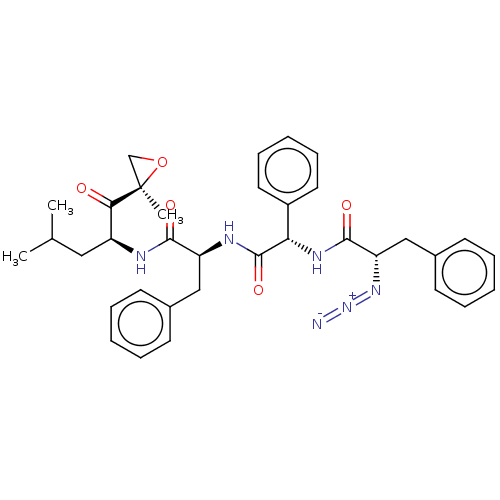 (CHEMBL4555159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536458 (CHEMBL4556673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536449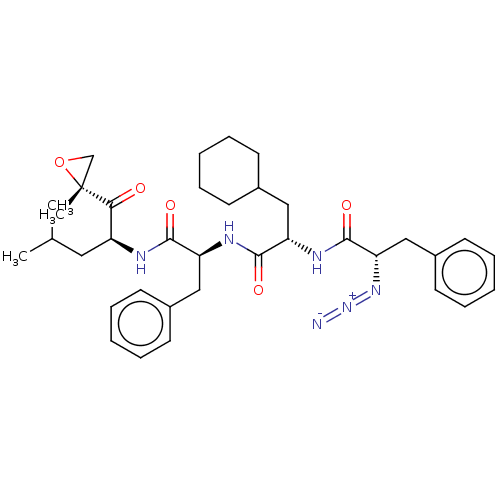 (CHEMBL4563141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536450 (CHEMBL4522697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536451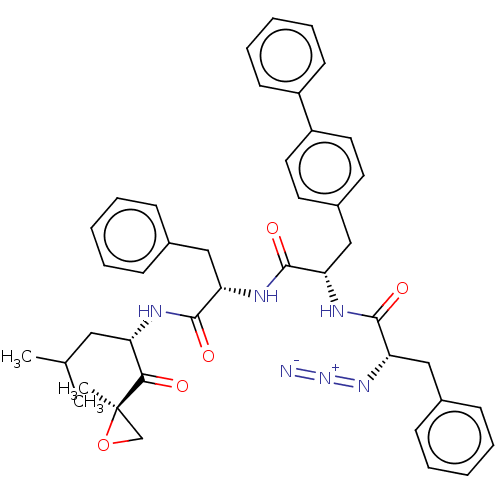 (CHEMBL4528476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536452 (CHEMBL4574722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536453 (CHEMBL4587878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536428 (CHEMBL4556631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536458 (CHEMBL4556673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536440 (CHEMBL4560006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536428 (CHEMBL4556631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536450 (CHEMBL4522697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536451 (CHEMBL4528476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536453 (CHEMBL4587878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536437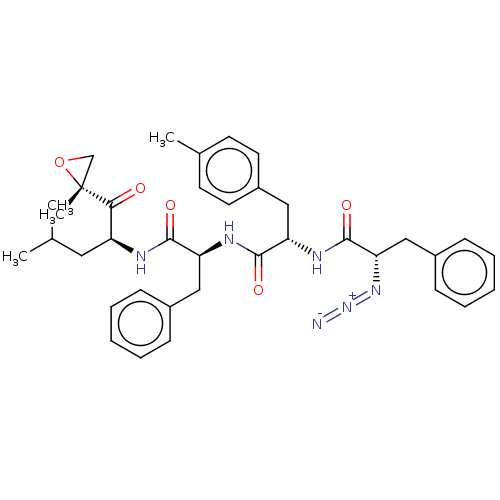 (CHEMBL4588350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536452 (CHEMBL4574722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536437 (CHEMBL4588350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536430 (CHEMBL4543267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536444 (CHEMBL4562003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536436 (CHEMBL4556581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536457 (CHEMBL4555159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536436 (CHEMBL4556581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100194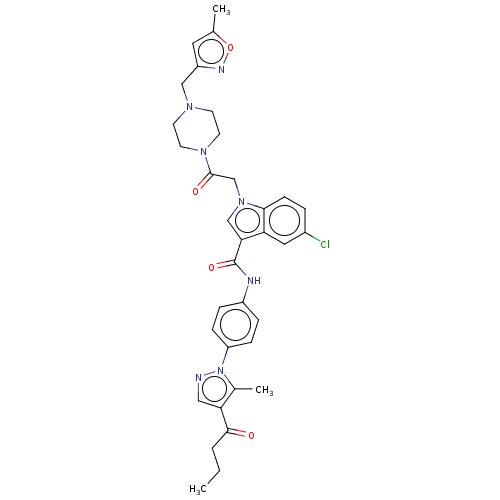 (CHEMBL3325804) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100197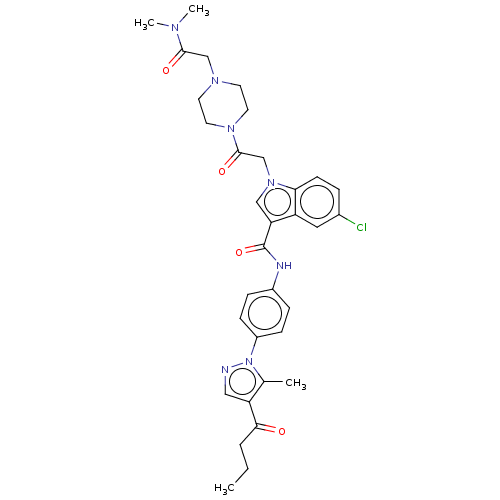 (CHEMBL3325803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 382 total ) | Next | Last >> |