 Found 72 hits with Last Name = 'beuming' and Initial = 't'
Found 72 hits with Last Name = 'beuming' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50131922
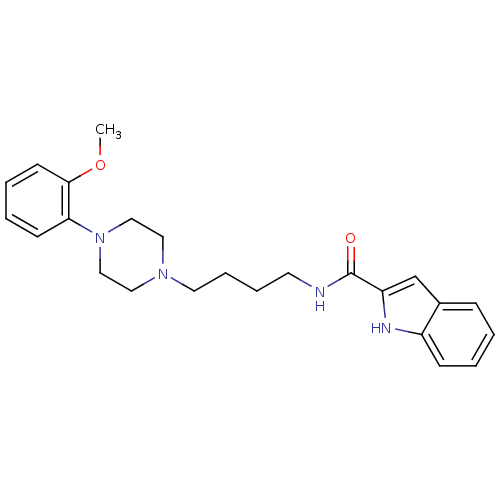
(1H-Indole-2-carboxylic acid {4-[4-(2-methoxy-pheny...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2cc3ccccc3[nH]2)CC1 Show InChI InChI=1S/C24H30N4O2/c1-30-23-11-5-4-10-22(23)28-16-14-27(15-17-28)13-7-6-12-25-24(29)21-18-19-8-2-3-9-20(19)26-21/h2-5,8-11,18,26H,6-7,12-17H2,1H3,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50122048
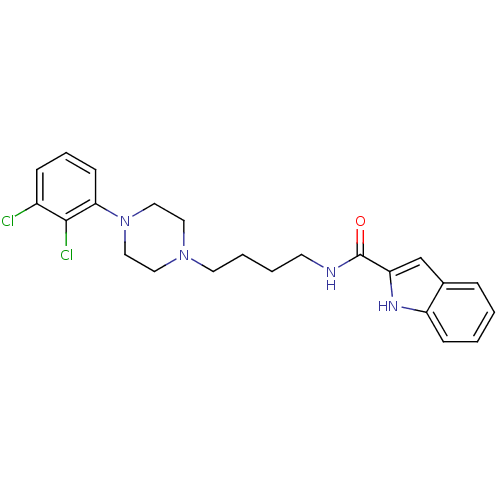
(1H-Indole-2-carboxylic acid {4-[4-(2,3-dichloro-ph...)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cc4ccccc4[nH]3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4O/c24-18-7-5-9-21(22(18)25)29-14-12-28(13-15-29)11-4-3-10-26-23(30)20-16-17-6-1-2-8-19(17)27-20/h1-2,5-9,16,27H,3-4,10-15H2,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394925
(CHEMBL2165677)Show InChI InChI=1S/C14H20Cl2N2/c1-2-3-7-17-8-10-18(11-9-17)13-6-4-5-12(15)14(13)16/h4-6H,2-3,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394925

(CHEMBL2165677)Show InChI InChI=1S/C14H20Cl2N2/c1-2-3-7-17-8-10-18(11-9-17)13-6-4-5-12(15)14(13)16/h4-6H,2-3,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394924
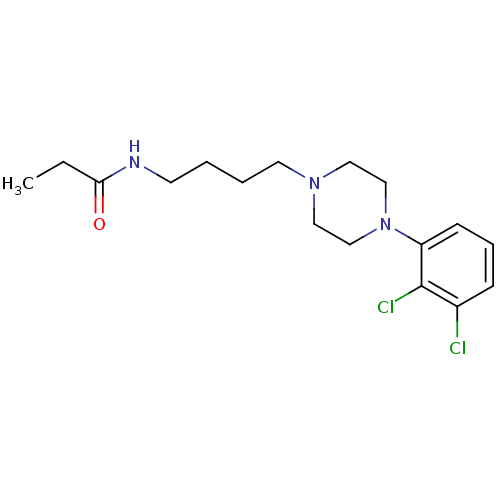
(CHEMBL2165678)Show InChI InChI=1S/C17H25Cl2N3O/c1-2-16(23)20-8-3-4-9-21-10-12-22(13-11-21)15-7-5-6-14(18)17(15)19/h5-7H,2-4,8-13H2,1H3,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394926
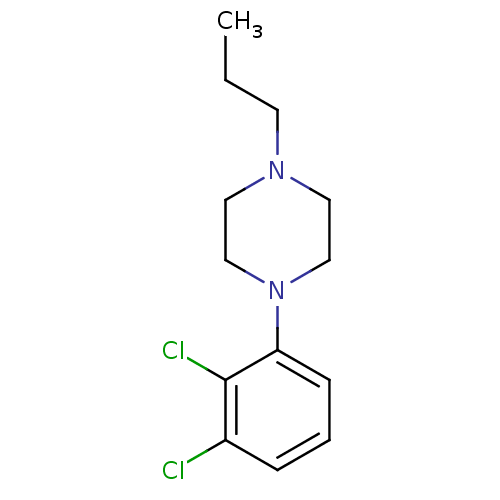
(CHEMBL2165676)Show InChI InChI=1S/C13H18Cl2N2/c1-2-6-16-7-9-17(10-8-16)12-5-3-4-11(14)13(12)15/h3-5H,2,6-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394926

(CHEMBL2165676)Show InChI InChI=1S/C13H18Cl2N2/c1-2-6-16-7-9-17(10-8-16)12-5-3-4-11(14)13(12)15/h3-5H,2,6-10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394924

(CHEMBL2165678)Show InChI InChI=1S/C17H25Cl2N3O/c1-2-16(23)20-8-3-4-9-21-10-12-22(13-11-21)15-7-5-6-14(18)17(15)19/h5-7H,2-4,8-13H2,1H3,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50039791

(1-Butyl-4-(2-methoxy-phenyl)-piperazine | CHEMBL26...)Show InChI InChI=1S/C15H24N2O/c1-3-4-9-16-10-12-17(13-11-16)14-7-5-6-8-15(14)18-2/h5-8H,3-4,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50039791

(1-Butyl-4-(2-methoxy-phenyl)-piperazine | CHEMBL26...)Show InChI InChI=1S/C15H24N2O/c1-3-4-9-16-10-12-17(13-11-16)14-7-5-6-8-15(14)18-2/h5-8H,3-4,9-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394927

(CHEMBL2165675)Show InChI InChI=1S/C12H16Cl2N2O/c13-10-2-1-3-11(12(10)14)16-6-4-15(5-7-16)8-9-17/h1-3,17H,4-9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50131922

(1H-Indole-2-carboxylic acid {4-[4-(2-methoxy-pheny...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2cc3ccccc3[nH]2)CC1 Show InChI InChI=1S/C24H30N4O2/c1-30-23-11-5-4-10-22(23)28-16-14-27(15-17-28)13-7-6-12-25-24(29)21-18-19-8-2-3-9-20(19)26-21/h2-5,8-11,18,26H,6-7,12-17H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394923

(CHEMBL2165679)Show InChI InChI=1S/C18H29N3O2/c1-3-18(22)19-10-6-7-11-20-12-14-21(15-13-20)16-8-4-5-9-17(16)23-2/h4-5,8-9H,3,6-7,10-15H2,1-2H3,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394929
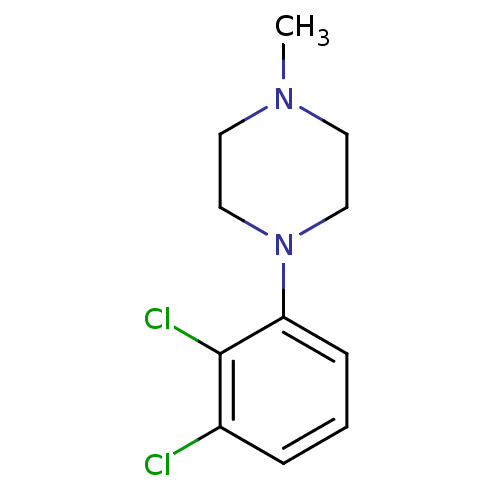
(CHEMBL2165673)Show InChI InChI=1S/C11H14Cl2N2/c1-14-5-7-15(8-6-14)10-4-2-3-9(12)11(10)13/h2-4H,5-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394929

(CHEMBL2165673)Show InChI InChI=1S/C11H14Cl2N2/c1-14-5-7-15(8-6-14)10-4-2-3-9(12)11(10)13/h2-4H,5-8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394927

(CHEMBL2165675)Show InChI InChI=1S/C12H16Cl2N2O/c13-10-2-1-3-11(12(10)14)16-6-4-15(5-7-16)8-9-17/h1-3,17H,4-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394923

(CHEMBL2165679)Show InChI InChI=1S/C18H29N3O2/c1-3-18(22)19-10-6-7-11-20-12-14-21(15-13-20)16-8-4-5-9-17(16)23-2/h4-5,8-9H,3,6-7,10-15H2,1-2H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50122048

(1H-Indole-2-carboxylic acid {4-[4-(2,3-dichloro-ph...)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cc4ccccc4[nH]3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4O/c24-18-7-5-9-21(22(18)25)29-14-12-28(13-15-29)11-4-3-10-26-23(30)20-16-17-6-1-2-8-19(17)27-20/h1-2,5-9,16,27H,3-4,10-15H2,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394930

(CHEMBL1424807)Show InChI InChI=1S/C10H12Cl2N2/c11-8-2-1-3-9(10(8)12)14-6-4-13-5-7-14/h1-3,13H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394930

(CHEMBL1424807)Show InChI InChI=1S/C10H12Cl2N2/c11-8-2-1-3-9(10(8)12)14-6-4-13-5-7-14/h1-3,13H,4-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394928

(CHEMBL2165674)Show InChI InChI=1S/C12H18N2O/c1-13-7-9-14(10-8-13)11-5-3-4-6-12(11)15-2/h3-6H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394928

(CHEMBL2165674)Show InChI InChI=1S/C12H18N2O/c1-13-7-9-14(10-8-13)11-5-3-4-6-12(11)15-2/h3-6H,7-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001862
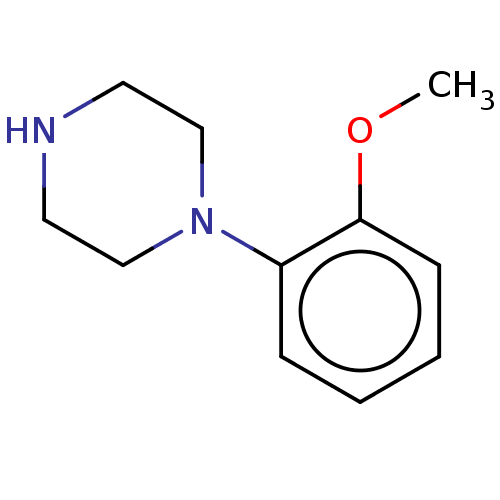
((N-(o-methoxyphenyl)piperazine)1-(2-Methoxy-phenyl...)Show InChI InChI=1S/C11H16N2O/c1-14-11-5-3-2-4-10(11)13-8-6-12-7-9-13/h2-5,12H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50001862

((N-(o-methoxyphenyl)piperazine)1-(2-Methoxy-phenyl...)Show InChI InChI=1S/C11H16N2O/c1-14-11-5-3-2-4-10(11)13-8-6-12-7-9-13/h2-5,12H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50394922

(CHEMBL2164434)Show InChI InChI=1S/C13H16N2O/c1-2-3-8-14-13(16)12-9-10-6-4-5-7-11(10)15-12/h4-7,9,15H,2-3,8H2,1H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D2L receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50394922

(CHEMBL2164434)Show InChI InChI=1S/C13H16N2O/c1-2-3-8-14-13(16)12-9-10-6-4-5-7-11(10)15-12/h4-7,9,15H,2-3,8H2,1H3,(H,14,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis |
J Med Chem 55: 6689-99 (2012)
Article DOI: 10.1021/jm300482h
BindingDB Entry DOI: 10.7270/Q2Q81F6B |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600764
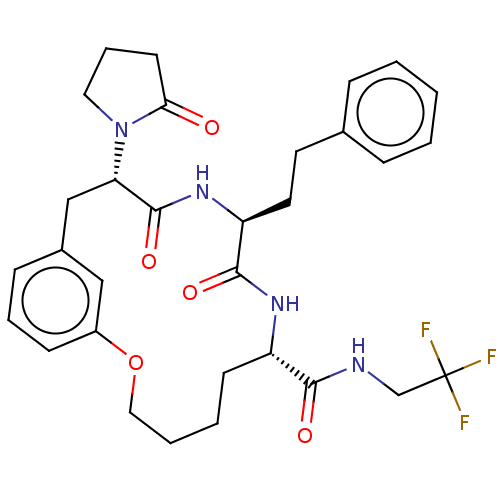
(CHEMBL5182017)Show SMILES FC(F)(F)CNC(=O)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600773
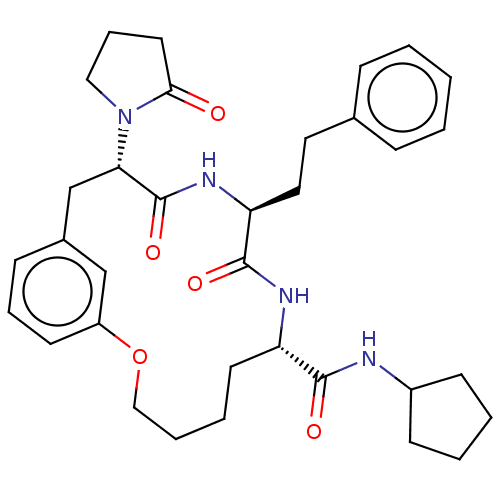
(CHEMBL5174759 | US20240043470, Compound 3-08)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600775
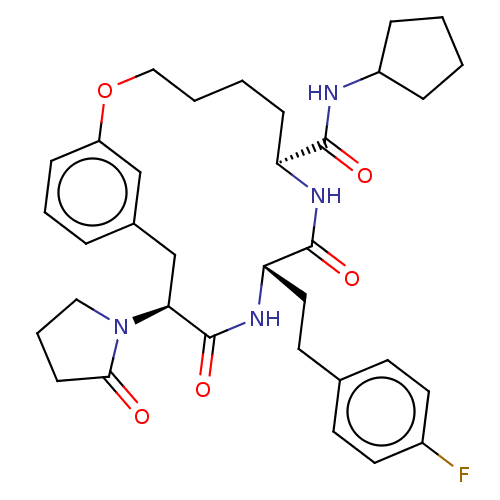
(CHEMBL5207942 | US20240043470, Compound 3-28)Show SMILES Fc1ccc(CC[C@@H]2NC(=O)[C@H](Cc3cccc(OCCCC[C@H](NC2=O)C(=O)NC2CCCC2)c3)N2CCCC2=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600767
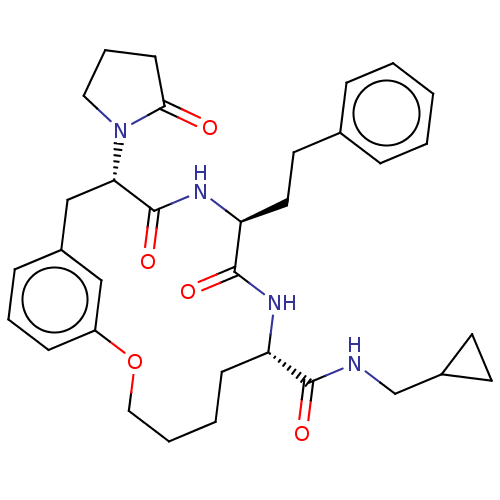
(CHEMBL5191353 | US20240043470, Compound 3-07)Show SMILES O=C(NCC1CC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600776

(CHEMBL5198676 | US20240043470, Compound 3-42)Show SMILES FC1(F)CCN(CC[C@@H]2NC(=O)[C@H](Cc3cccc(OCCCC[C@H](NC2=O)C(=O)NC2CCCC2)c3)N2CCCC2=O)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600762

(CHEMBL5180427 | US20240043470, Compound 1-53)Show SMILES CC(=O)N[C@H]1Cc2cccc(Oc3cccc(C[C@H](NC(=O)[C@H](CCc4ccccc4)NC1=O)C(=O)NCC(F)(F)F)c3)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600765
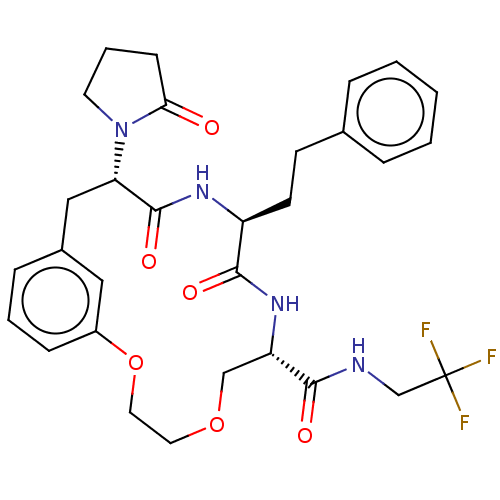
(CHEMBL5175549 | US20240043470, Compound 3-09)Show SMILES FC(F)(F)CNC(=O)[C@@H]1COCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600766

(CHEMBL5193963 | US20240043470, Compound 3-10)Show SMILES FC(F)CNC(=O)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600768
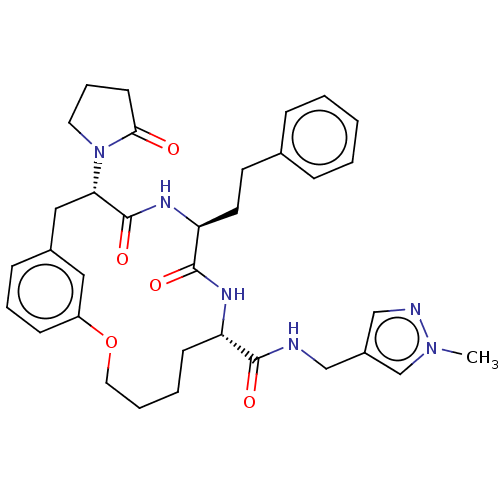
(CHEMBL5195365 | US20240043470, Compound 3-11)Show SMILES Cn1cc(CNC(=O)[C@@H]2CCCCOc3cccc(C[C@H](N4CCCC4=O)C(=O)N[C@@H](CCc4ccccc4)C(=O)N2)c3)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600774

(CHEMBL5200145 | US20240043470, Compound 3-35)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](COc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600782
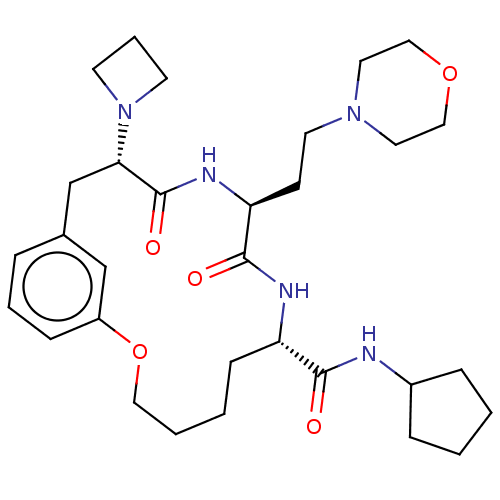
(CHEMBL5198080)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCC3)C(=O)N[C@@H](CCN3CCOCC3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50600782

(CHEMBL5198080)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCC3)C(=O)N[C@@H](CCN3CCOCC3)C(=O)N1)c2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600763
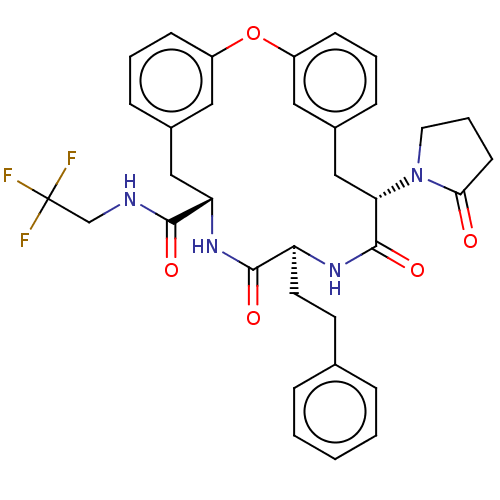
(CHEMBL5194112 | US20240043470, Compound 1-56)Show SMILES FC(F)(F)CNC(=O)[C@@H]1Cc2cccc(Oc3cccc(C[C@H](N4CCCC4=O)C(=O)N[C@@H](CCc4ccccc4)C(=O)N1)c3)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600779
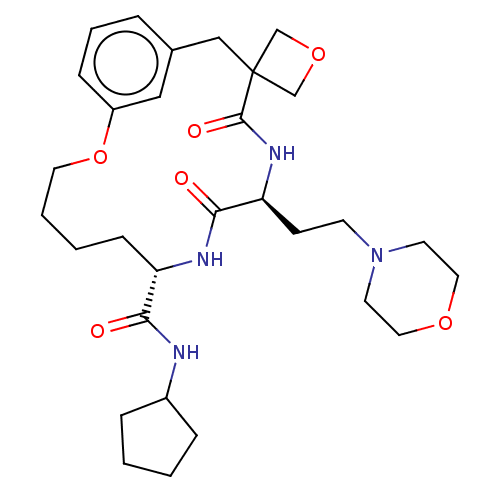
(CHEMBL5198924)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(CC3(COC3)C(=O)N[C@@H](CCN3CCOCC3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600778
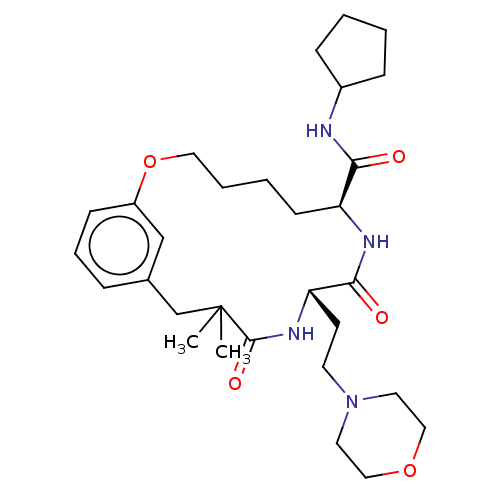
(CHEMBL5198102 | US20240043470, Compound 3-40)Show SMILES CC1(C)Cc2cccc(OCCCC[C@H](NC(=O)[C@H](CCN3CCOCC3)NC1=O)C(=O)NC1CCCC1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50600776

(CHEMBL5198676 | US20240043470, Compound 3-42)Show SMILES FC1(F)CCN(CC[C@@H]2NC(=O)[C@H](Cc3cccc(OCCCC[C@H](NC2=O)C(=O)NC2CCCC2)c3)N2CCCC2=O)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50600775

(CHEMBL5207942 | US20240043470, Compound 3-28)Show SMILES Fc1ccc(CC[C@@H]2NC(=O)[C@H](Cc3cccc(OCCCC[C@H](NC2=O)C(=O)NC2CCCC2)c3)N2CCCC2=O)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600772
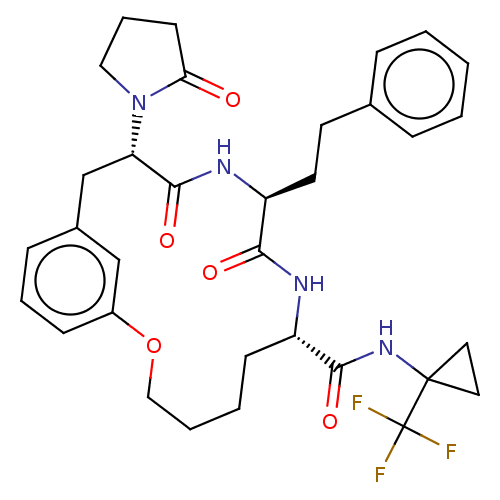
(CHEMBL5179439 | US20240043470, Compound 3-15)Show SMILES FC(F)(F)C1(CC1)NC(=O)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600777
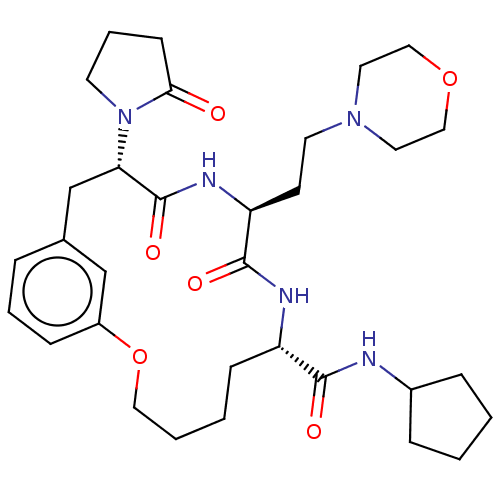
(CHEMBL5173314 | US20240043470, Compound 3-20)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCN3CCOCC3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600780
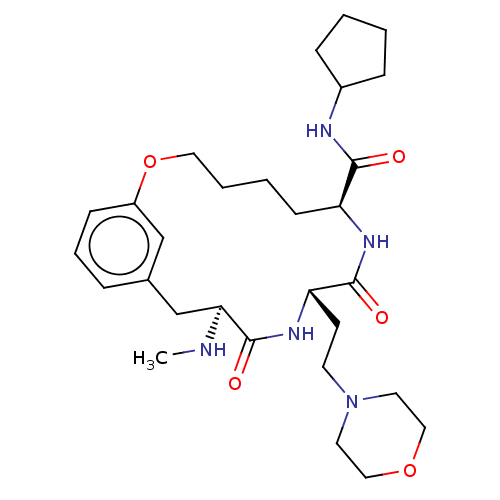
(CHEMBL5174268)Show SMILES CN[C@H]1Cc2cccc(OCCCC[C@H](NC(=O)[C@H](CCN3CCOCC3)NC1=O)C(=O)NC1CCCC1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600781
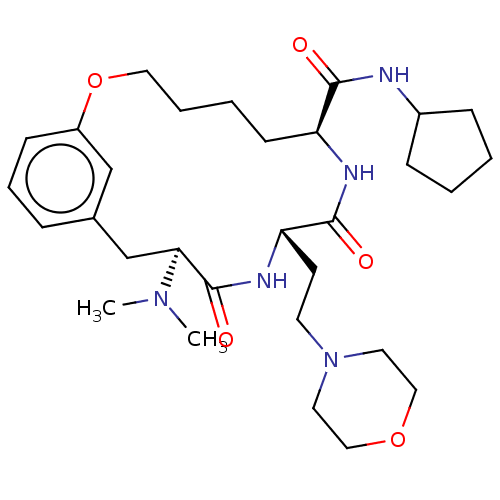
(CHEMBL5187232 | US20240043470, Compound 3-34)Show SMILES CN(C)[C@H]1Cc2cccc(OCCCC[C@H](NC(=O)[C@H](CCN3CCOCC3)NC1=O)C(=O)NC1CCCC1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50587102
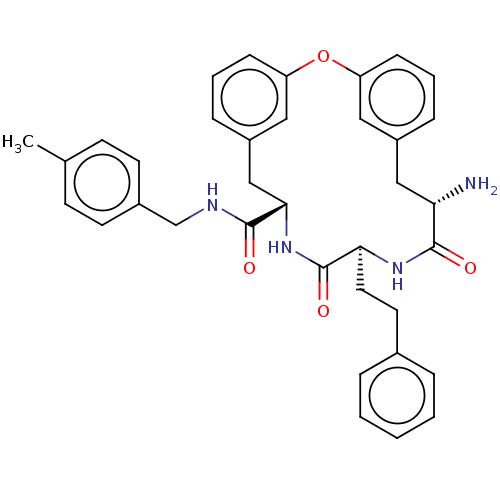
(CHEMBL5081872 | US20240043470, Compound 1-69)Show SMILES Cc1ccc(CNC(=O)[C@@H]2Cc3cccc(Oc4cccc(C[C@H](N)C(=O)N[C@@H](CCc5ccccc5)C(=O)N2)c4)c3)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50600761

(CHEMBL5197086 | US20240043470, Compound 1-51)Show SMILES CC(=O)N[C@H]1Cc2cccc(Oc3cccc(C[C@H](NC(=O)[C@H](CCc4ccccc4)NC1=O)C(=O)NCc1ccc(C)cc1)c3)c2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50600762

(CHEMBL5180427 | US20240043470, Compound 1-53)Show SMILES CC(=O)N[C@H]1Cc2cccc(Oc3cccc(C[C@H](NC(=O)[C@H](CCc4ccccc4)NC1=O)C(=O)NCC(F)(F)F)c3)c2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data