

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM686 (BMS-186318 analog 23 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM680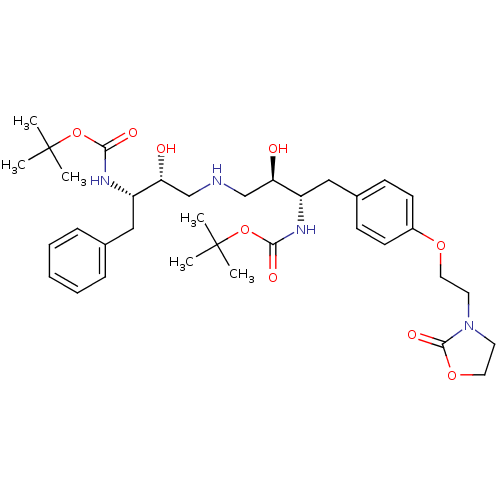 ((phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM679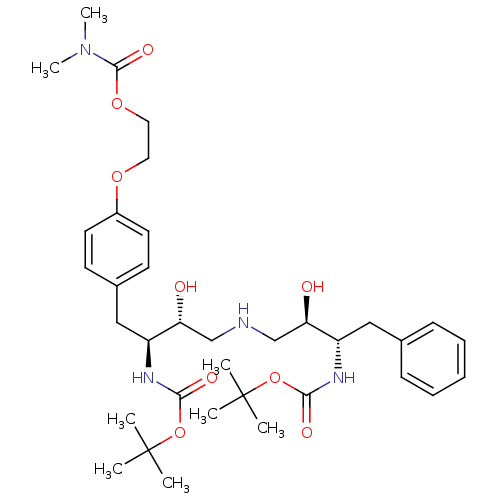 (BMS-186318 analog 16 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM676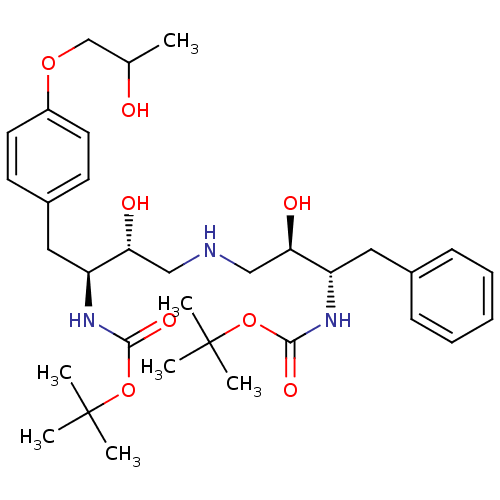 (BMS-186318 analog 13 | [1S-[1R*, 2S*(2S*, 3R*)]-[3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM691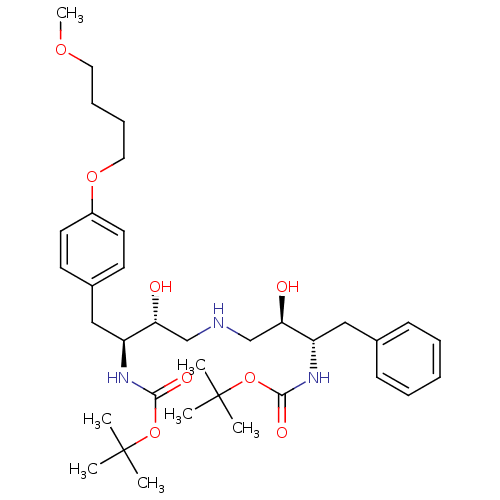 (BMS-186318 analog 28 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM671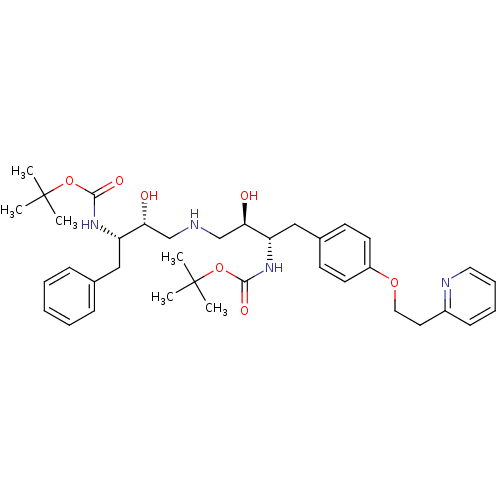 (BMS-186318 analog 8 | [1S-[1R*,2S*(2S*,3R*)]]-[3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM681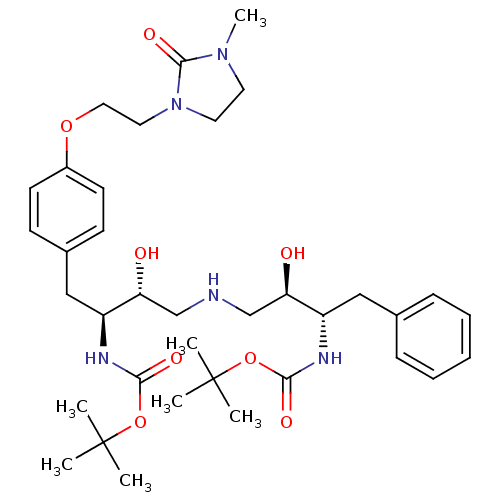 (BMS-186318 analog 18 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM672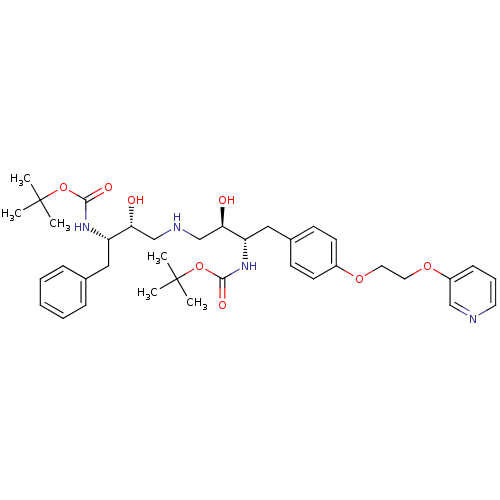 ((phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM674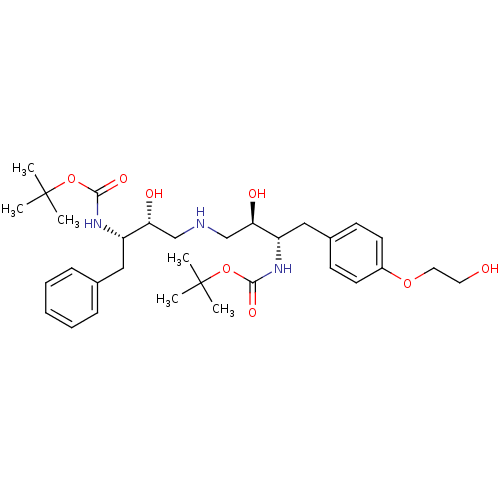 (BMS-186318 analog 11 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM675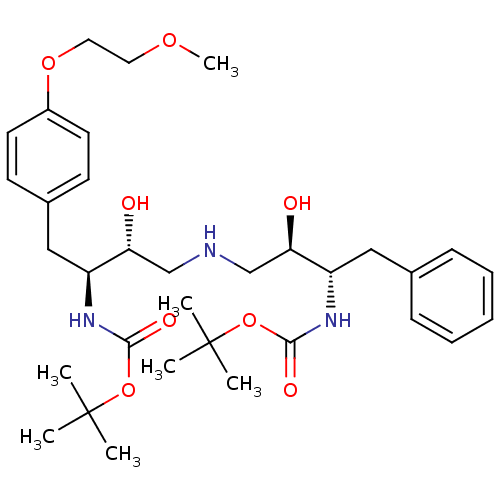 (BMS-186318 analog 12 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM693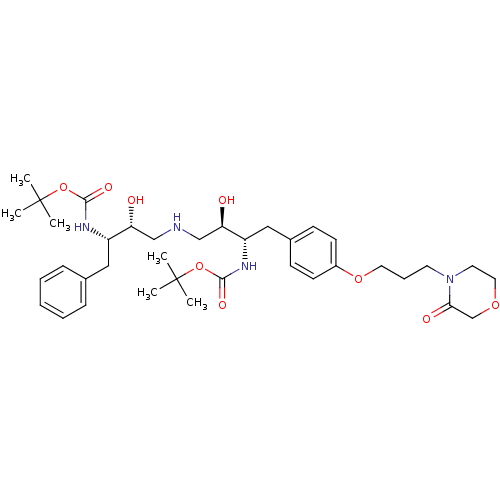 (BMS-186318 analog 30 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM683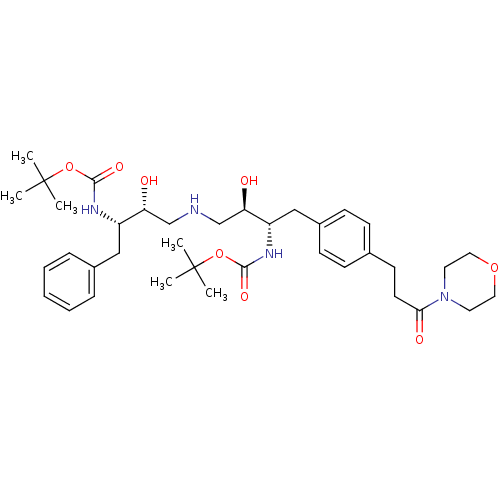 (BMS-186318 analog 20 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM692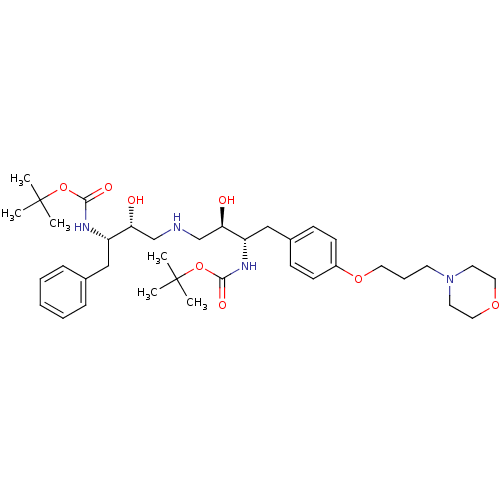 (BMS-186318 analog 29 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM685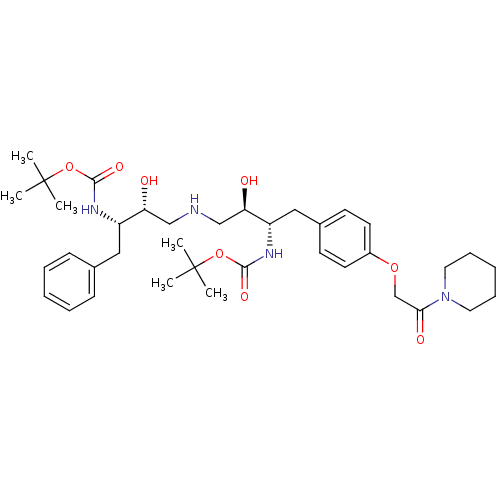 (BMS-186318 analog 22 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM670 (BMS-186318 analog 7 | [1S-[1R*,2S*(2S*,3R*)]]-[3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM684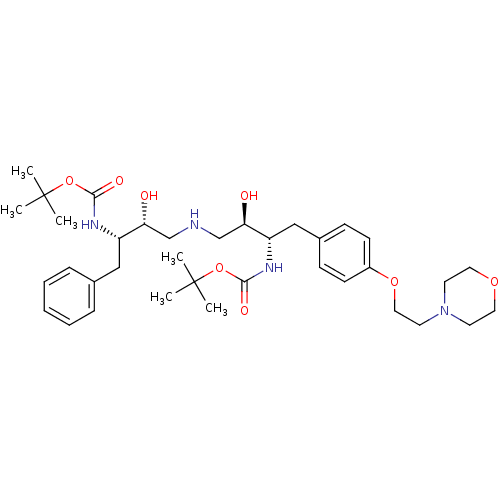 ((phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM682 (BMS-186318 analog 19 | [1S-[1R*,2S*(2S*,3R*)]]-[3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM918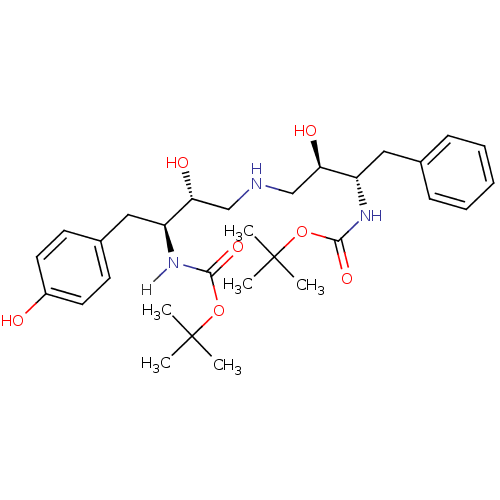 ((1S-(1R*,2S*(2S,3R*)))-[3-[[3-[[(1,1-Dimethylethox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM673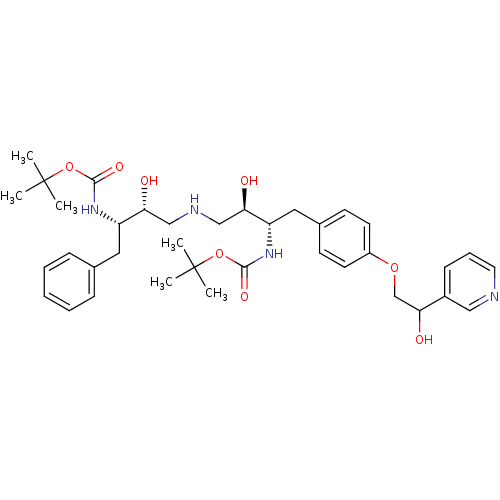 ((phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM677 ((phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM914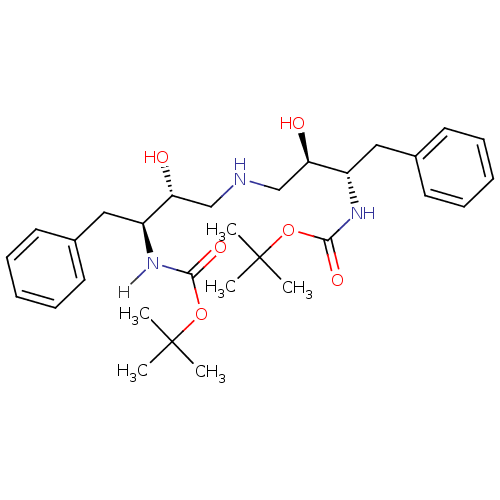 (Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM666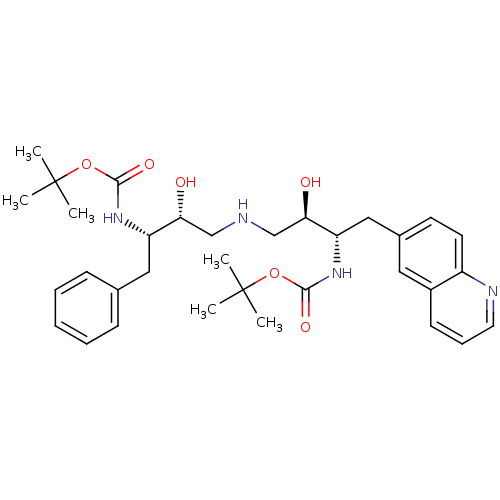 (BMS-186318 analog 3 | [1S-[1R*,2S*(2S*,3R*)]]-[3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM688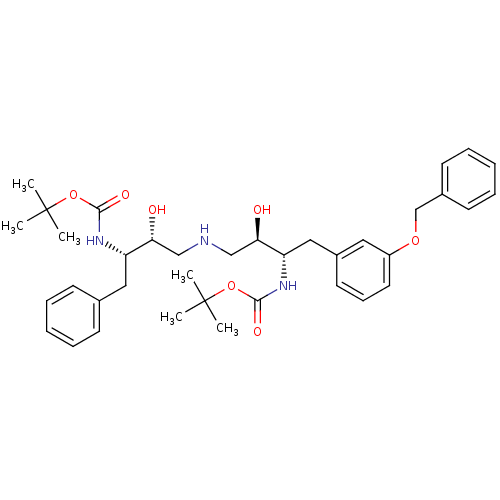 (BMS-186318 analog 25 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM678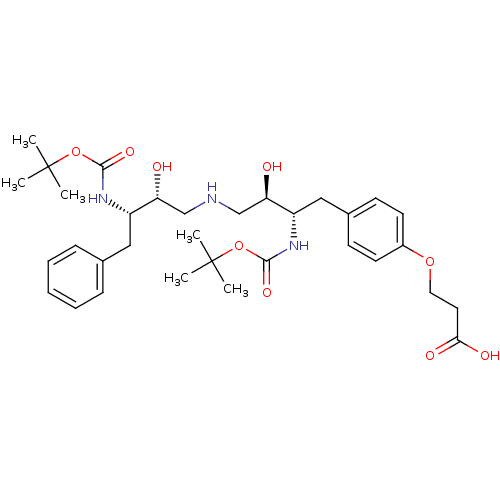 (3-{4-[(2S,3R)-2-{[(tert-butoxy)carbonyl]amino}-4-{...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM668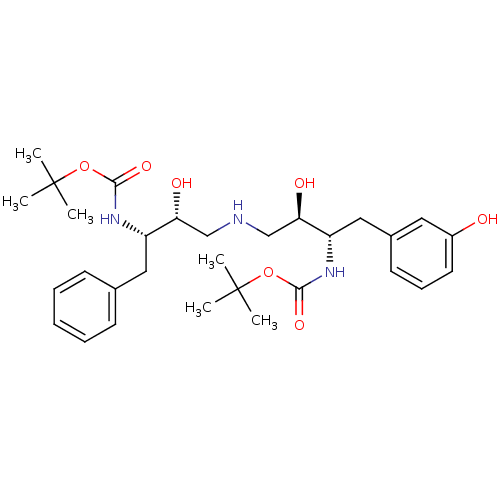 (BMS-186318 analog 5 | [1S-[1R*,2S*(2S*,3R*)]]-[3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM689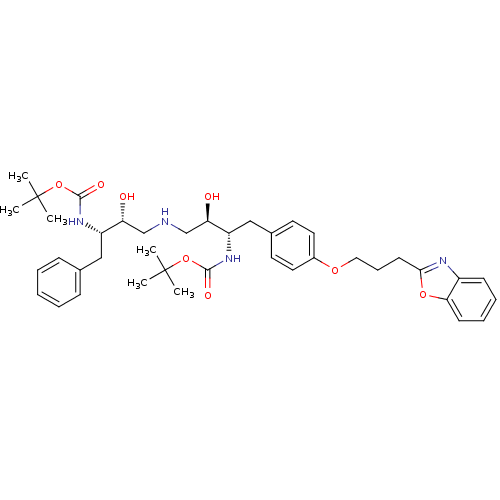 (BMS-186318 analog 26 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM669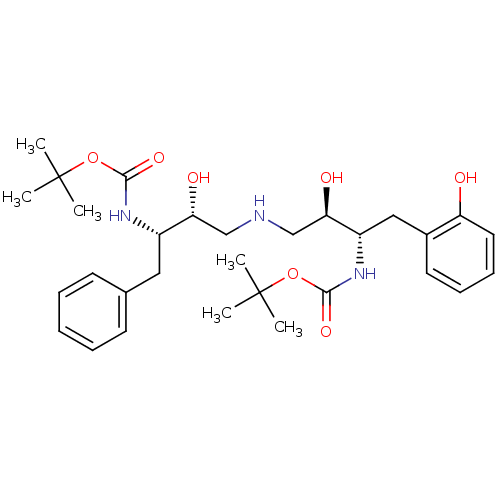 (BMS-186318 analog 6 | [1S-[1R*,2S*(2S*,3R*)]]-[3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM687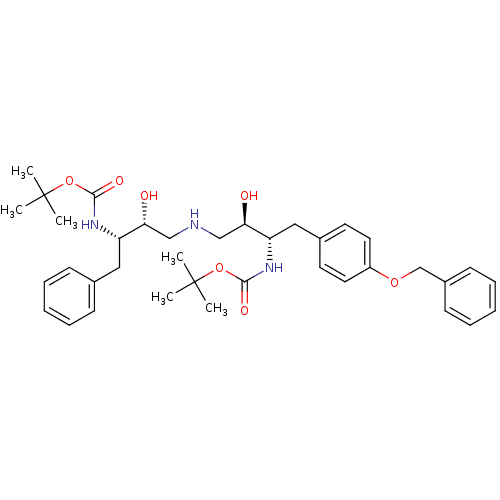 (BMS-186318 analog 24 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM690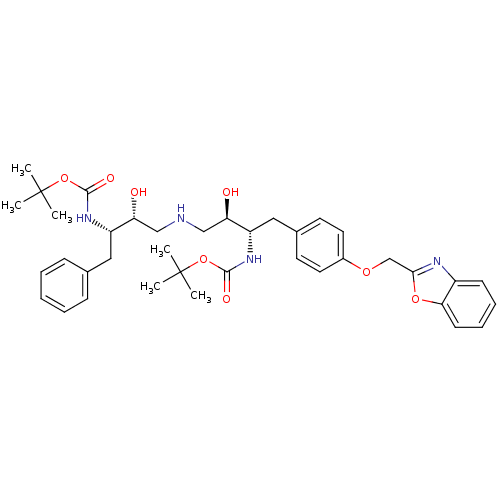 (BMS-186318 analog 27 | tert-butyl N-[(2S,3R)-4-{[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM665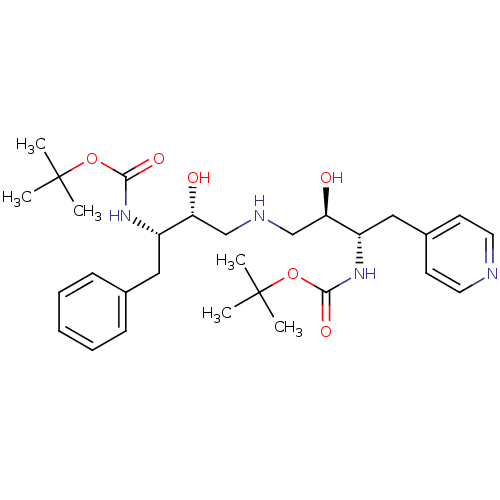 (BMS-186318 analog 2 | [1S-[1R*,2S*(2S*,3R*)]]-[3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Bristol-Myers Squibb Company | Assay Description IC50 values are determined by inhibition of the cleavage of the peptidic substrate [V-S-Q-N-(beta-naphthylalanine)-P-I-V]. The IC50 value represents ... | J Med Chem 39: 1991-2007 (1996) Article DOI: 10.1021/jm950717a BindingDB Entry DOI: 10.7270/Q2VX0DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205383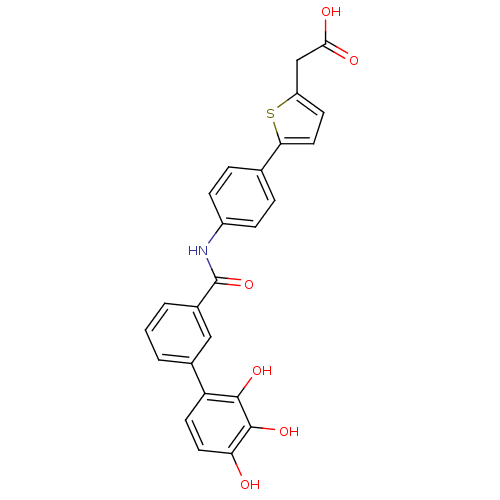 ((5-{4-[(2',3',4'-trihydroxy-biphenyl-3-carbonyl)-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205368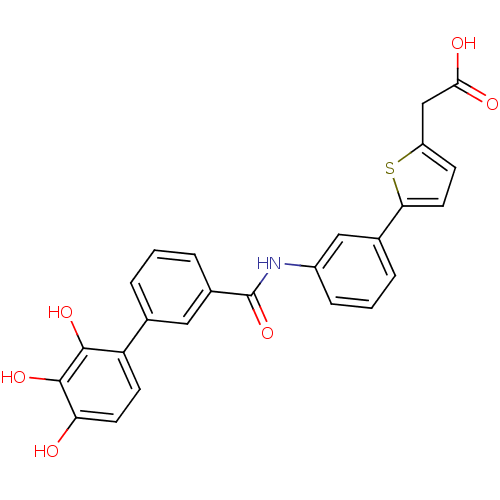 ((5-{3-[(2',3',4'-trihydroxy-biphenyl-3-carbonyl)-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205383 ((5-{4-[(2',3',4'-trihydroxy-biphenyl-3-carbonyl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50205396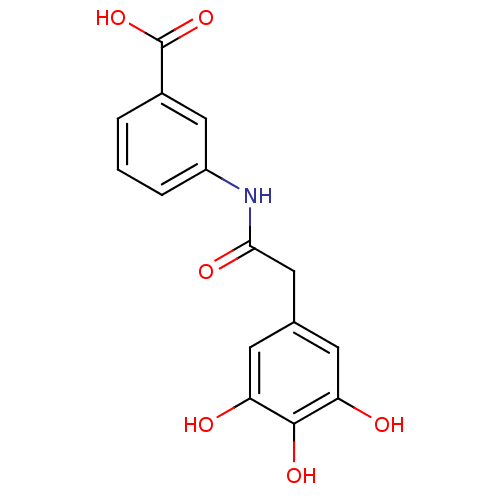 (3-[2-(3,4,5-trihydroxy-phenyl)-acetylamino]-benzoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human E-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205377 (CHEMBL220254 | {2'-[(3,4,5-trihydroxybenzoyl)oxy]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50205385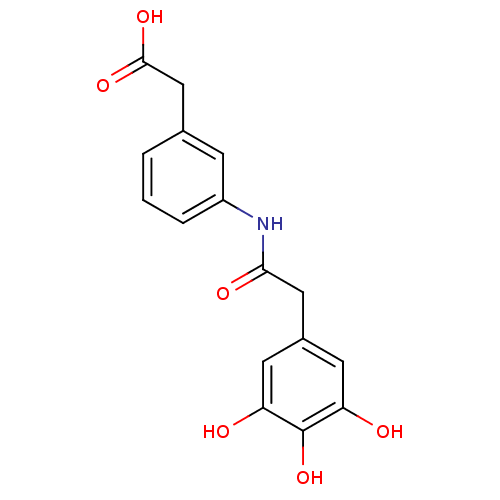 (CHEMBL220255 | {3-[2-(3,4,5-trihydroxy-phenyl)-ace...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human E-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205394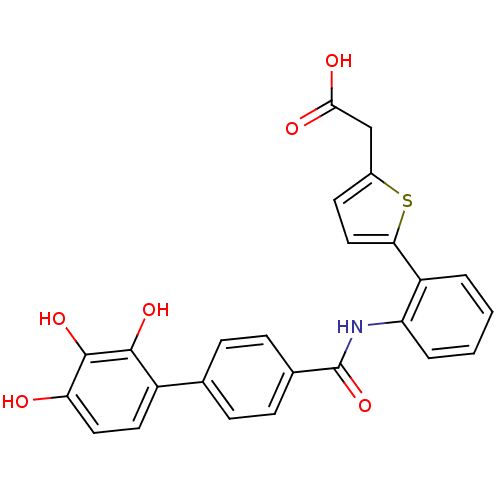 ((5-{2-[(2',3',4'-trihydroxy-biphenyl-4-carbonyl)-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205368 ((5-{3-[(2',3',4'-trihydroxy-biphenyl-3-carbonyl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205396 (3-[2-(3,4,5-trihydroxy-phenyl)-acetylamino]-benzoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205403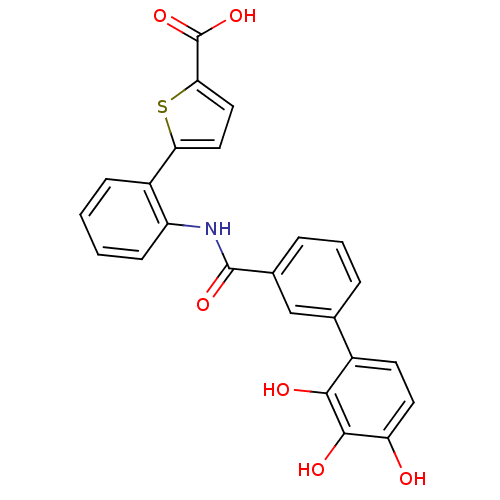 (5-{2-[(2'3',4'-hydroxy-biphenyl-3-carbonyl)-amino]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50205371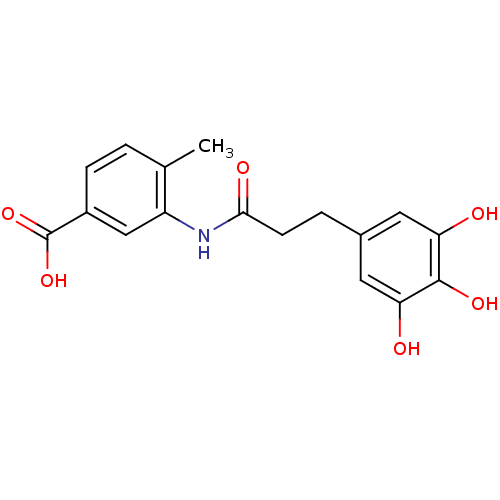 (4-methyl-3-[3-(3,4,5-trihydroxy-phenyl)-propionyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human E-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205396 (3-[2-(3,4,5-trihydroxy-phenyl)-acetylamino]-benzoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205377 (CHEMBL220254 | {2'-[(3,4,5-trihydroxybenzoyl)oxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205394 ((5-{2-[(2',3',4'-trihydroxy-biphenyl-4-carbonyl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205403 (5-{2-[(2'3',4'-hydroxy-biphenyl-3-carbonyl)-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205385 (CHEMBL220255 | {3-[2-(3,4,5-trihydroxy-phenyl)-ace...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205375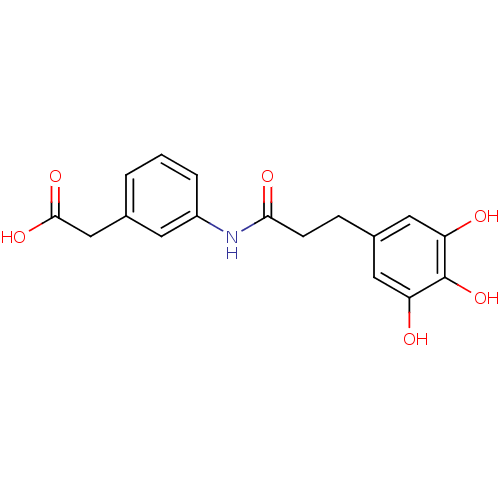 (CHEMBL220419 | {3-[3-(3,4,5-trihydroxy-phenyl)-pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50205375 (CHEMBL220419 | {3-[3-(3,4,5-trihydroxy-phenyl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human L-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50205375 (CHEMBL220419 | {3-[3-(3,4,5-trihydroxy-phenyl)-pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human E-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50205384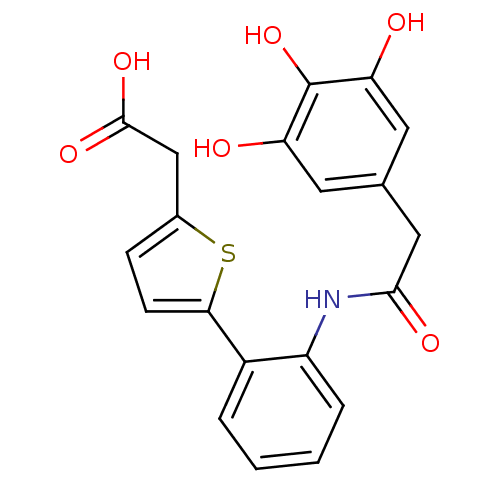 ((5-{2-[2-(3,4,5-trihydroxy-phenyl)-acetylamino]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Revotar Biopharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human P-selectin after 2 hrs | J Med Chem 50: 1101-15 (2007) Article DOI: 10.1021/jm060536g BindingDB Entry DOI: 10.7270/Q2TB17Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |