

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50263384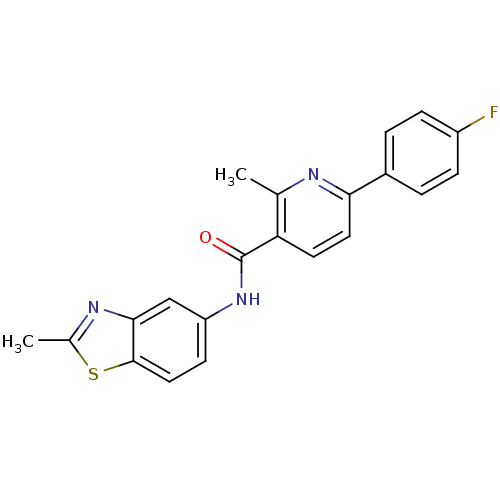 (6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 5.3 | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by whole cell patch clamp a... | Bioorg Med Chem Lett 18: 5609-13 (2008) Article DOI: 10.1016/j.bmcl.2008.08.105 BindingDB Entry DOI: 10.7270/Q2PZ58PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429204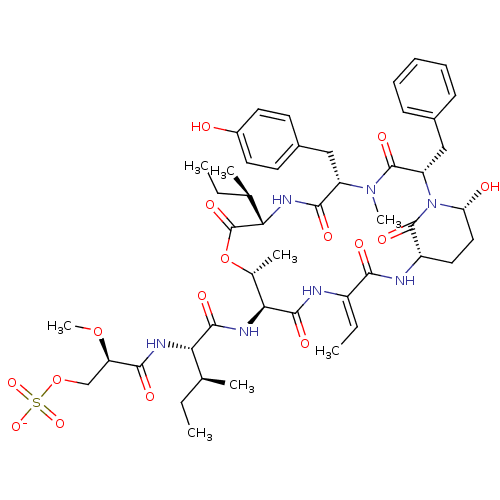 (CHEMBL2338549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429202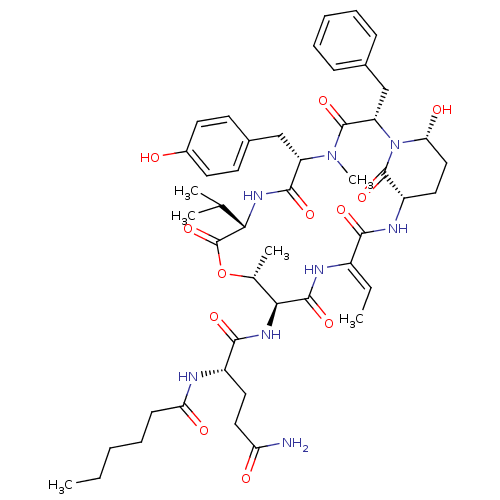 (LYNGBYASTATIN 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429205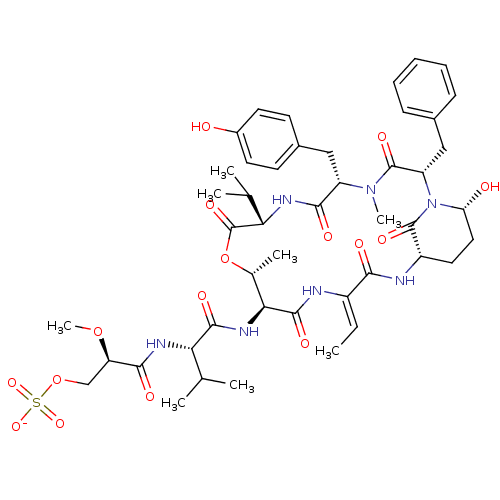 (CHEMBL2338551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50263384 (6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.3 | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by FLIPR assay | Bioorg Med Chem Lett 18: 5609-13 (2008) Article DOI: 10.1016/j.bmcl.2008.08.105 BindingDB Entry DOI: 10.7270/Q2PZ58PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429206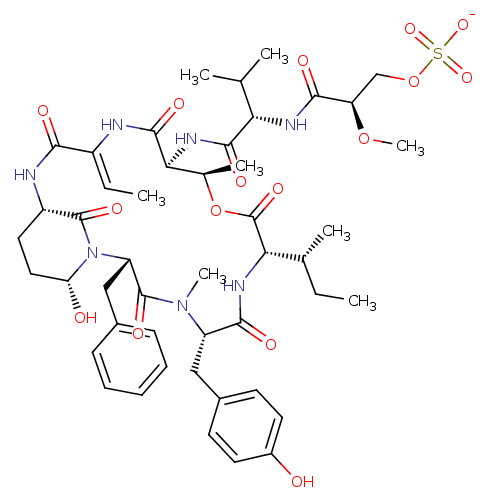 (CHEMBL2338550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50263384 (6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced calcium influx by whole cell patch clamp ass... | Bioorg Med Chem Lett 18: 5609-13 (2008) Article DOI: 10.1016/j.bmcl.2008.08.105 BindingDB Entry DOI: 10.7270/Q2PZ58PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429203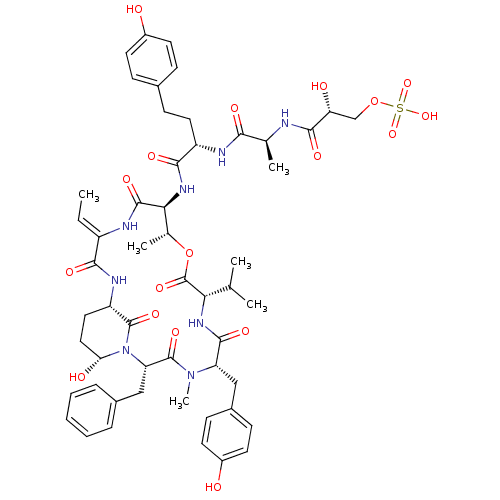 (LYNGBYASTATIN 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Cavia porcellus) | BDBM50263384 (6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 5.3 | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at guinea pig TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by FLIPR assay | Bioorg Med Chem Lett 18: 5609-13 (2008) Article DOI: 10.1016/j.bmcl.2008.08.105 BindingDB Entry DOI: 10.7270/Q2PZ58PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429201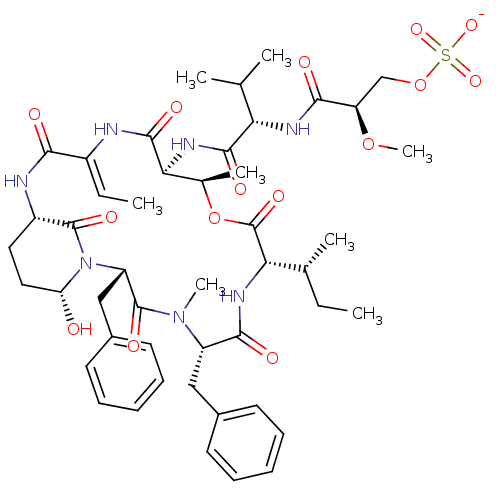 (CHEMBL2338554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-induced antiproliferative activity expressed in BEAS2B cells assessed as cytoprotectivity after 24 hrs by MTT... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50263384 (6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 5.3 | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by FLIPR assay | Bioorg Med Chem Lett 18: 5609-13 (2008) Article DOI: 10.1016/j.bmcl.2008.08.105 BindingDB Entry DOI: 10.7270/Q2PZ58PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429208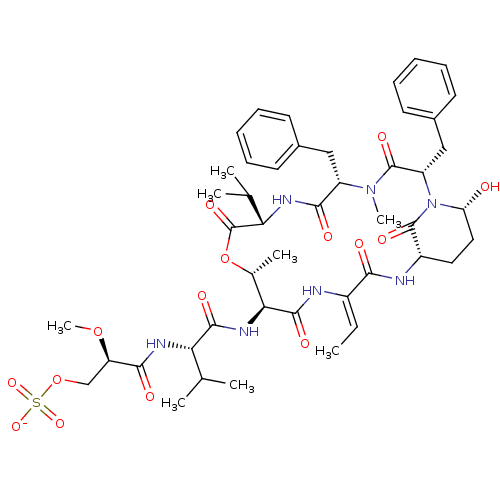 (CHEMBL2338553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084637 (2,2-Dimethyl-propionic acid 4-[2-(carboxymethyl-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429201 (CHEMBL2338554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50429207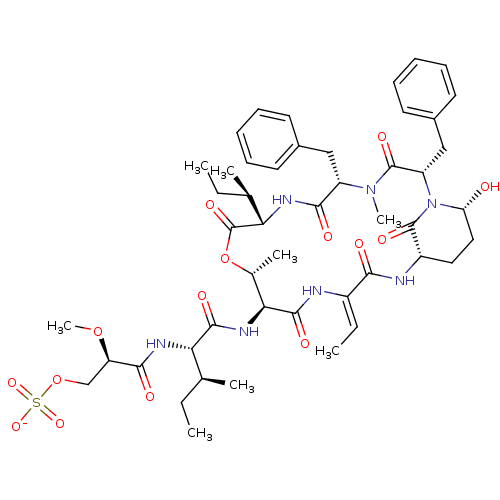 (CHEMBL2338552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase assessed as proteolysis using N-(methoxysuccinyl)-Ala-Ala-Pro-Val-p-nitroanilide substrate incubated for 15 m... | J Med Chem 56: 1276-90 (2013) Article DOI: 10.1021/jm3017305 BindingDB Entry DOI: 10.7270/Q2CR5VQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263425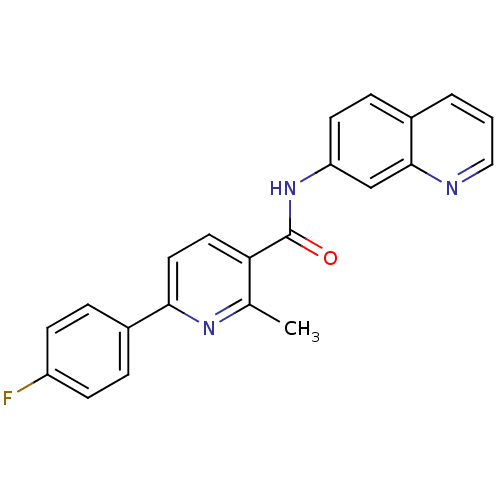 (6-(4-fluorophenyl)-2-methyl-N-(quinolin-7-yl)nicot...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | Bioorg Med Chem Lett 18: 5609-13 (2008) Article DOI: 10.1016/j.bmcl.2008.08.105 BindingDB Entry DOI: 10.7270/Q2PZ58PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||