 Found 79 hits with Last Name = 'boggetto' and Initial = 'n'
Found 79 hits with Last Name = 'boggetto' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156909
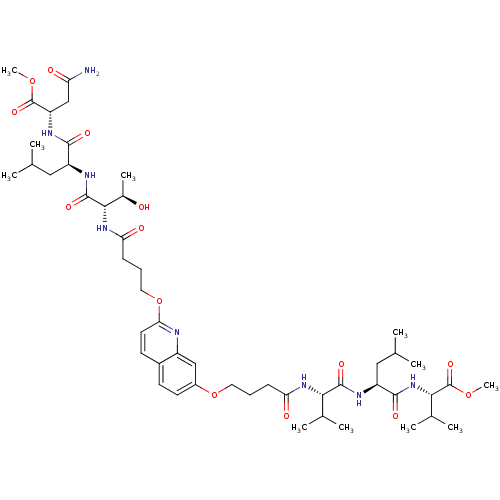
(CHEMBL376817 | methyl (2S)-3-carbamoyl-2-[(2S)-2-[...)Show SMILES COC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2n1)[C@@H](C)O |r| Show InChI InChI=1S/C49H76N8O14/c1-26(2)22-34(44(62)54-36(25-37(50)59)48(66)68-10)53-47(65)43(30(9)58)56-39(61)15-13-21-71-40-19-17-31-16-18-32(24-33(31)51-40)70-20-12-14-38(60)55-41(28(5)6)46(64)52-35(23-27(3)4)45(63)57-42(29(7)8)49(67)69-11/h16-19,24,26-30,34-36,41-43,58H,12-15,20-23,25H2,1-11H3,(H2,50,59)(H,52,64)(H,53,65)(H,54,62)(H,55,60)(H,56,61)(H,57,63)/t30-,34+,35+,36+,41+,42+,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463
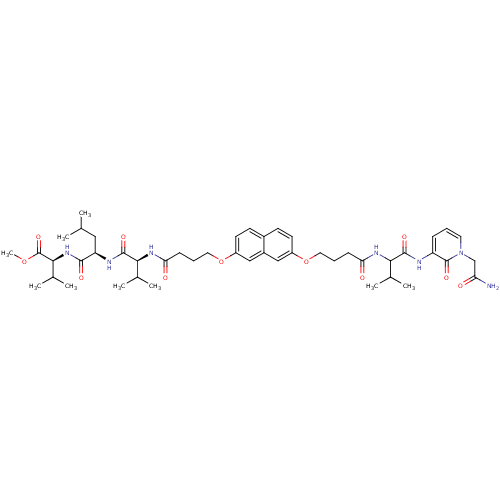
(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease 150V mutant |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156906
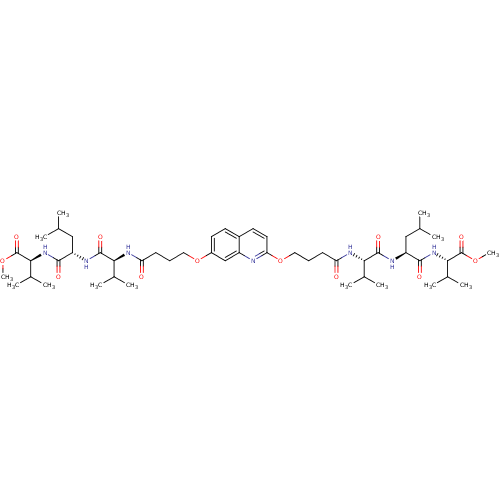
(CHEMBL375050 | methyl (2S)-2-[(2S)-2-[(2S)-2-(4-{[...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)nc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C51H81N7O12/c1-28(2)25-37(46(61)57-44(32(9)10)50(65)67-13)53-48(63)42(30(5)6)55-39(59)17-15-23-69-35-21-19-34-20-22-41(52-36(34)27-35)70-24-16-18-40(60)56-43(31(7)8)49(64)54-38(26-29(3)4)47(62)58-45(33(11)12)51(66)68-14/h19-22,27-33,37-38,42-45H,15-18,23-26H2,1-14H3,(H,53,63)(H,54,64)(H,55,59)(H,56,60)(H,57,61)(H,58,62)/t37-,38-,42-,43-,44-,45-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191464
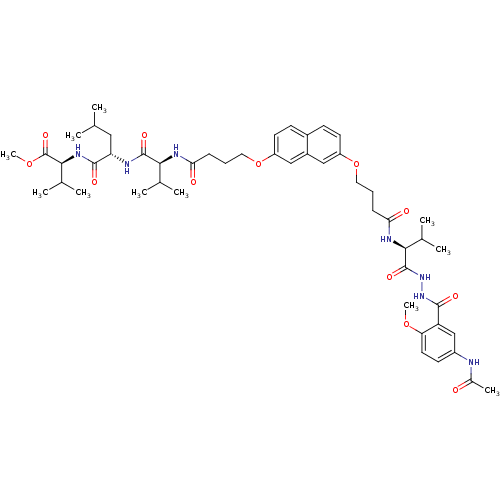
(CHEMBL212749 | methyl (2S)-2-[(2S)-2-[(2S)-2-(4-{[...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)NNC(=O)c3cc(NC(C)=O)ccc3OC)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C50H71N7O12/c1-28(2)24-39(47(62)55-45(31(7)8)50(65)67-11)52-48(63)43(29(3)4)53-41(59)14-12-22-68-36-19-16-33-17-20-37(26-34(33)25-36)69-23-13-15-42(60)54-44(30(5)6)49(64)57-56-46(61)38-27-35(51-32(9)58)18-21-40(38)66-10/h16-21,25-31,39,43-45H,12-15,22-24H2,1-11H3,(H,51,58)(H,52,63)(H,53,59)(H,54,60)(H,55,62)(H,56,61)(H,57,64)/t39-,43-,44-,45-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease dimerization |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463

(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease ANAM-11 mutant |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156901
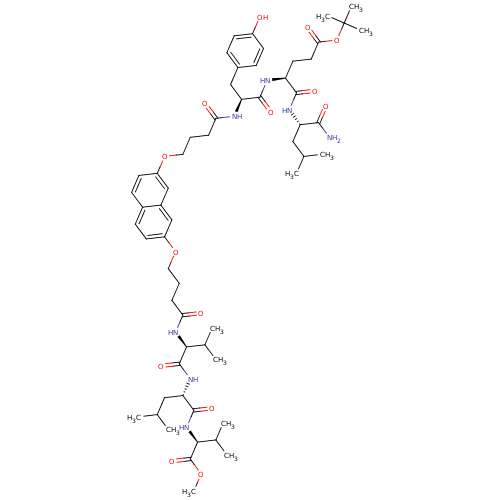
((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)cc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C59H87N7O14/c1-34(2)29-45(53(60)71)63-54(72)44(25-26-50(70)80-59(9,10)11)62-55(73)47(31-38-17-21-41(67)22-18-38)61-48(68)15-13-27-78-42-23-19-39-20-24-43(33-40(39)32-42)79-28-14-16-49(69)65-51(36(5)6)57(75)64-46(30-35(3)4)56(74)66-52(37(7)8)58(76)77-12/h17-24,32-37,44-47,51-52,67H,13-16,25-31H2,1-12H3,(H2,60,71)(H,61,68)(H,62,73)(H,63,72)(H,64,75)(H,65,69)(H,66,74)/t44-,45-,46-,47-,51-,52-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463

(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease V82A mutant |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50051763
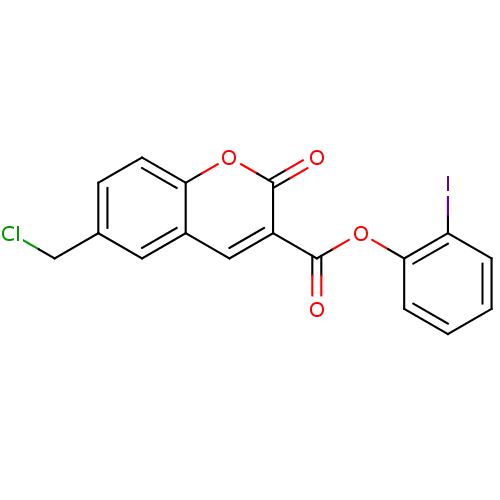
(2-iodophenyl 6-(chloromethyl)-2-oxo-2H-chromene-3-...)Show InChI InChI=1S/C17H10ClIO4/c18-9-10-5-6-14-11(7-10)8-12(16(20)22-14)17(21)23-15-4-2-1-3-13(15)19/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156902
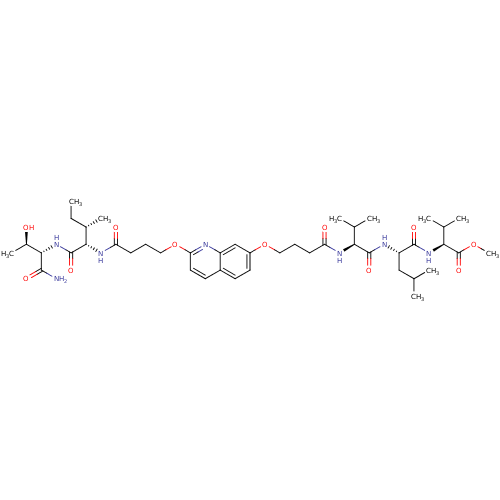
((S)-methyl 2-((S)-2-((S)-2-(4-(2-(4-((2S,3S)-1-((2...)Show SMILES CC[C@H](C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2n1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C44H69N7O11/c1-11-27(8)38(43(58)51-39(28(9)52)40(45)55)49-34(54)15-13-21-62-35-19-17-29-16-18-30(23-31(29)46-35)61-20-12-14-33(53)48-36(25(4)5)42(57)47-32(22-24(2)3)41(56)50-37(26(6)7)44(59)60-10/h16-19,23-28,32,36-39,52H,11-15,20-22H2,1-10H3,(H2,45,55)(H,47,57)(H,48,53)(H,49,54)(H,50,56)(H,51,58)/t27-,28+,32-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM23563

(3-Carboxylate-coumarin deriv., 3 | CHEMBL13357 | p...)Show InChI InChI=1S/C17H11ClO4/c18-10-11-6-7-15-12(8-11)9-14(17(20)22-15)16(19)21-13-4-2-1-3-5-13/h1-9H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50051762

(6-Chloromethyl-2-oxo-2H-chromene-3-carboxylic acid...)Show InChI InChI=1S/C18H13ClO4/c1-11-4-2-3-5-15(11)22-17(20)14-9-13-8-12(10-19)6-7-16(13)23-18(14)21/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156903
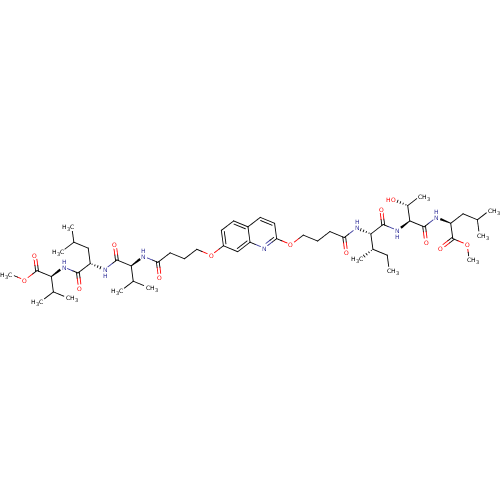
((S)-methyl 2-((2S,3R)-3-hydroxy-2-((2S,3S)-2-(4-(7...)Show SMILES CC[C@H](C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2n1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)OC |r| Show InChI InChI=1S/C51H81N7O13/c1-14-32(10)44(48(64)58-45(33(11)59)49(65)54-38(26-29(4)5)50(66)68-12)56-40(61)18-16-24-71-41-22-20-34-19-21-35(27-36(34)52-41)70-23-15-17-39(60)55-42(30(6)7)47(63)53-37(25-28(2)3)46(62)57-43(31(8)9)51(67)69-13/h19-22,27-33,37-38,42-45,59H,14-18,23-26H2,1-13H3,(H,53,63)(H,54,65)(H,55,60)(H,56,61)(H,57,62)(H,58,64)/t32-,33+,37-,38-,42-,43-,44-,45-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50135844

((2S,8S)-2-[({[({[(1S)-1-{[(1S,2R)-2-(benzyloxy)-1-...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2C3CC=C4C[C@@H](CC[C@]4(C)C3CC[C@]12C)SC[C@@H]1CCN2CC[C@@H](CSCC(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)OCc3ccccc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc3ccccc3)C(=O)OCc3ccccc3)NC2=[NH+]1 |c:108,t:14| Show InChI InChI=1S/C80H116N10O10S2/c1-50(2)20-19-21-52(5)63-30-31-64-62-29-28-58-42-61(32-36-79(58,8)65(62)33-37-80(63,64)9)102-48-60-35-39-90-38-34-59(84-78(90)85-60)47-101-49-71(93)82-44-70(92)83-53(6)73(94)89-72(54(7)99-45-56-24-15-11-16-25-56)76(97)87-66(40-51(3)4)74(95)86-67(43-69(81)91)75(96)88-68(41-55-22-13-10-14-23-55)77(98)100-46-57-26-17-12-18-27-57/h10-18,22-28,50-54,59-68,72H,19-21,29-49H2,1-9H3,(H2,81,91)(H,82,93)(H,83,92)(H,84,85)(H,86,95)(H,87,97)(H,88,96)(H,89,94)/p+1/t52-,53+,54-,59+,60+,61-,62?,63-,64?,65?,66+,67+,68+,72+,79+,80-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid
Curated by ChEMBL
| Assay Description
Competitive inhibition against HIV-1 Protease |
J Med Chem 46: 5196-207 (2003)
Article DOI: 10.1021/jm030871u
BindingDB Entry DOI: 10.7270/Q2C53MKZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463

(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease dimerization |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156910
((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)nc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C58H86N8O14/c1-33(2)29-43(52(59)71)63-53(72)41(24-26-49(70)80-58(9,10)11)62-54(73)45(31-37-17-21-39(67)22-18-37)60-46(68)15-14-28-79-48-25-20-38-19-23-40(32-42(38)61-48)78-27-13-16-47(69)65-50(35(5)6)56(75)64-44(30-34(3)4)55(74)66-51(36(7)8)57(76)77-12/h17-23,25,32-36,41,43-45,50-51,67H,13-16,24,26-31H2,1-12H3,(H2,59,71)(H,60,68)(H,62,73)(H,63,72)(H,64,75)(H,65,69)(H,66,74)/t41-,43-,44-,45-,50-,51-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156908
((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)cc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C50H78N6O12/c1-29(2)25-38(45(51)60)53-46(61)37(21-22-42(59)68-50(9,10)11)52-40(57)15-13-23-66-35-19-17-33-18-20-36(28-34(33)27-35)67-24-14-16-41(58)55-43(31(5)6)48(63)54-39(26-30(3)4)47(62)56-44(32(7)8)49(64)65-12/h17-20,27-32,37-39,43-44H,13-16,21-26H2,1-12H3,(H2,51,60)(H,52,57)(H,53,61)(H,54,63)(H,55,58)(H,56,62)/t37-,38-,39-,43-,44-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463

(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease D30N mutant |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463

(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease G48V/L90M mutant |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156904
((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)nc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C49H77N7O12/c1-28(2)25-36(44(50)60)53-45(61)34(20-22-41(59)68-49(9,10)11)51-38(57)15-14-24-67-40-21-18-32-17-19-33(27-35(32)52-40)66-23-13-16-39(58)55-42(30(5)6)47(63)54-37(26-29(3)4)46(62)56-43(31(7)8)48(64)65-12/h17-19,21,27-31,34,36-37,42-43H,13-16,20,22-26H2,1-12H3,(H2,50,60)(H,51,57)(H,53,61)(H,54,63)(H,55,58)(H,56,62)/t34-,36-,37-,42-,43-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50074687
(2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C52H82N6O12/c1-29(2)25-39(47(61)57-45(33(9)10)51(65)67-13)53-49(63)43(31(5)6)55-41(59)17-15-23-69-37-21-19-35-20-22-38(28-36(35)27-37)70-24-16-18-42(60)56-44(32(7)8)50(64)54-40(26-30(3)4)48(62)58-46(34(11)12)52(66)68-14/h19-22,27-34,39-40,43-46H,15-18,23-26H2,1-14H3,(H,53,63)(H,54,64)(H,55,59)(H,56,60)(H,57,61)(H,58,62)/t39-,40-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50051761
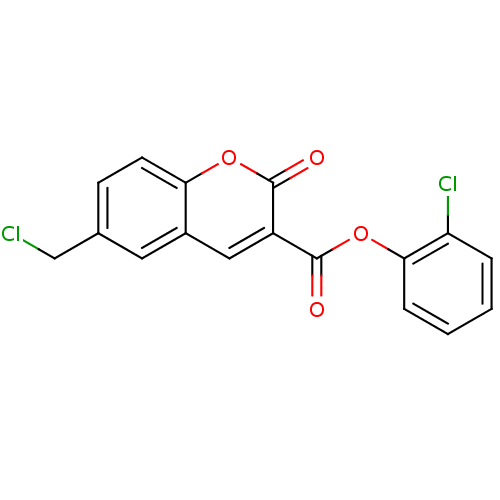
(2-chlorophenyl 6-(chloromethyl)-2-oxo-2H-chromene-...)Show InChI InChI=1S/C17H10Cl2O4/c18-9-10-5-6-14-11(7-10)8-12(16(20)22-14)17(21)23-15-4-2-1-3-13(15)19/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156911
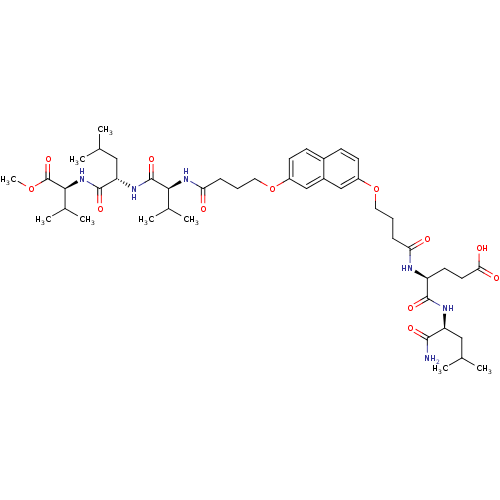
((S)-5-((S)-1-amino-4-methyl-1-oxopentan-2-ylamino)...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O)cc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C46H70N6O12/c1-26(2)22-35(42(47)57)49-43(58)34(18-19-39(55)56)48-37(53)12-10-20-63-32-16-14-30-15-17-33(25-31(30)24-32)64-21-11-13-38(54)51-40(28(5)6)45(60)50-36(23-27(3)4)44(59)52-41(29(7)8)46(61)62-9/h14-17,24-29,34-36,40-41H,10-13,18-23H2,1-9H3,(H2,47,57)(H,48,53)(H,49,58)(H,50,60)(H,51,54)(H,52,59)(H,55,56)/t34-,35-,36-,40-,41-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156905

((S)-methyl 2-((2S,3R)-3-hydroxy-2-(4-(7-(4-((S)-1-...)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)[C@@H](C)O |r| Show InChI InChI=1S/C46H71N5O12/c1-26(2)22-35(42(55)51-40(29(7)8)46(59)61-11)47-43(56)39(28(5)6)49-37(53)14-12-20-62-33-18-16-31-17-19-34(25-32(31)24-33)63-21-13-15-38(54)50-41(30(9)52)44(57)48-36(23-27(3)4)45(58)60-10/h16-19,24-30,35-36,39-41,52H,12-15,20-23H2,1-11H3,(H,47,56)(H,48,57)(H,49,53)(H,50,54)(H,51,55)/t30-,35+,36+,39+,40+,41+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156914
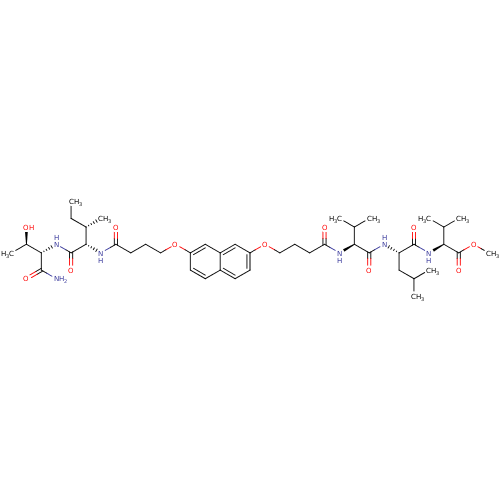
((S)-methyl 2-((S)-2-((S)-2-(4-(7-(4-((2S,3S)-1-((2...)Show SMILES CC[C@H](C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C45H70N6O11/c1-11-28(8)39(44(58)51-40(29(9)52)41(46)55)49-36(54)15-13-21-62-33-19-17-30-16-18-32(23-31(30)24-33)61-20-12-14-35(53)48-37(26(4)5)43(57)47-34(22-25(2)3)42(56)50-38(27(6)7)45(59)60-10/h16-19,23-29,34,37-40,52H,11-15,20-22H2,1-10H3,(H2,46,55)(H,47,57)(H,48,53)(H,49,54)(H,50,56)(H,51,58)/t28-,29+,34-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156913
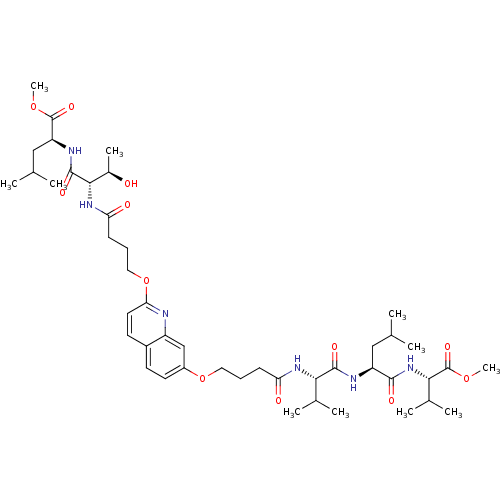
((S)-methyl 2-((2S,3R)-3-hydroxy-2-(4-(7-(4-((S)-1-...)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2n1)[C@@H](C)O |r| Show InChI InChI=1S/C45H70N6O12/c1-25(2)22-33(41(55)51-39(28(7)8)45(59)61-11)47-42(56)38(27(5)6)49-35(53)14-12-20-62-31-18-16-30-17-19-37(46-32(30)24-31)63-21-13-15-36(54)50-40(29(9)52)43(57)48-34(23-26(3)4)44(58)60-10/h16-19,24-29,33-34,38-40,52H,12-15,20-23H2,1-11H3,(H,47,56)(H,48,57)(H,49,53)(H,50,54)(H,51,55)/t29-,33+,34+,38+,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156912
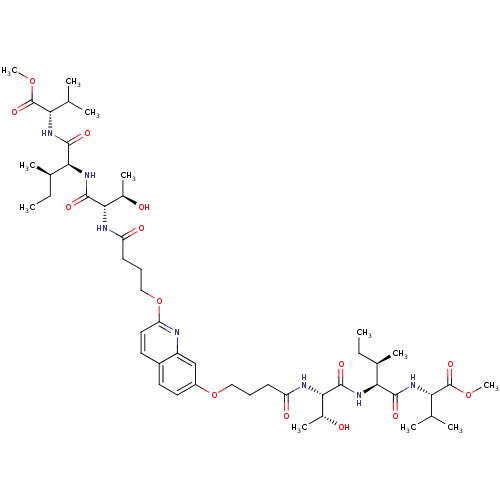
(CHEMBL376602 | methyl (2S)-2-[(2S,3R)-2-[(2S,3R)-3...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@H](C)CC)C(=O)N[C@@H](C(C)C)C(=O)OC)nc2c1)[C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C49H77N7O14/c1-13-28(7)40(44(61)53-38(26(3)4)48(65)67-11)55-46(63)42(30(9)57)51-35(59)17-15-23-69-33-21-19-32-20-22-37(50-34(32)25-33)70-24-16-18-36(60)52-43(31(10)58)47(64)56-41(29(8)14-2)45(62)54-39(27(5)6)49(66)68-12/h19-22,25-31,38-43,57-58H,13-18,23-24H2,1-12H3,(H,51,59)(H,52,60)(H,53,61)(H,54,62)(H,55,63)(H,56,64)/t28-,29-,30-,31-,38+,39+,40+,41+,42+,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156907
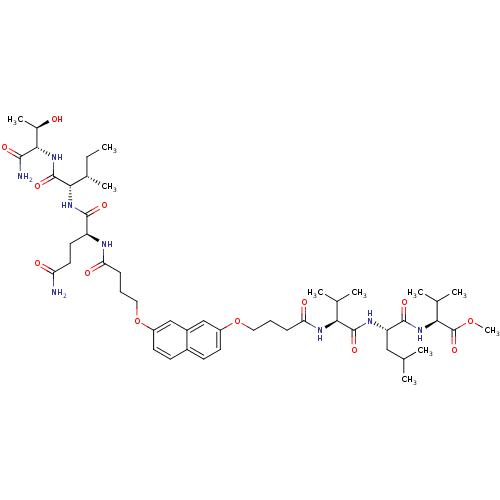
((S)-methyl 2-((S)-2-((S)-2-(4-(7-(4-((S)-5-amino-1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C50H78N8O13/c1-11-30(8)43(49(67)58-44(31(9)59)45(52)63)57-46(64)36(20-21-38(51)60)53-39(61)14-12-22-70-34-18-16-32-17-19-35(26-33(32)25-34)71-23-13-15-40(62)55-41(28(4)5)48(66)54-37(24-27(2)3)47(65)56-42(29(6)7)50(68)69-10/h16-19,25-31,36-37,41-44,59H,11-15,20-24H2,1-10H3,(H2,51,60)(H2,52,63)(H,53,61)(H,54,66)(H,55,62)(H,56,65)(H,57,64)(H,58,67)/t30-,31+,36-,37-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156902

((S)-methyl 2-((S)-2-((S)-2-(4-(2-(4-((2S,3S)-1-((2...)Show SMILES CC[C@H](C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2n1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C44H69N7O11/c1-11-27(8)38(43(58)51-39(28(9)52)40(45)55)49-34(54)15-13-21-62-35-19-17-29-16-18-30(23-31(29)46-35)61-20-12-14-33(53)48-36(25(4)5)42(57)47-32(22-24(2)3)41(56)50-37(26(6)7)44(59)60-10/h16-19,23-28,32,36-39,52H,11-15,20-22H2,1-10H3,(H2,45,55)(H,47,57)(H,48,53)(H,49,54)(H,50,56)(H,51,58)/t27-,28+,32-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191463

(CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NC(C(C)C)C(=O)Nc3cccn(CC(N)=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C47H67N7O11/c1-27(2)23-36(43(58)53-42(30(7)8)47(62)63-9)50-45(60)41(29(5)6)52-39(57)15-12-22-65-34-19-17-31-16-18-33(24-32(31)25-34)64-21-11-14-38(56)51-40(28(3)4)44(59)49-35-13-10-20-54(46(35)61)26-37(48)55/h10,13,16-20,24-25,27-30,36,40-42H,11-12,14-15,21-23,26H2,1-9H3,(H2,48,55)(H,49,59)(H,50,60)(H,51,56)(H,52,57)(H,53,58)/t36-,40?,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of dimerization of HIV1 protease MDR-HM mutant |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50135845
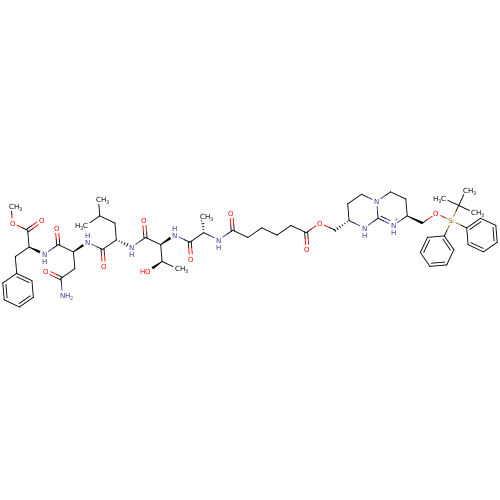
(Bicyclic Guanidinium Subunit | CHEMBL266754)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CCCCC(=O)OC[C@@H]1CCN2CC[C@@H](CO[Si](c3ccccc3)(c3ccccc3)C(C)(C)C)[NH+]=C2N1)[C@@H](C)O |c:77| Show InChI InChI=1S/C58H83N9O12Si/c1-37(2)32-45(53(73)63-46(34-48(59)69)54(74)65-47(56(76)77-8)33-40-20-12-9-13-21-40)64-55(75)51(39(4)68)66-52(72)38(3)60-49(70)26-18-19-27-50(71)78-35-41-28-30-67-31-29-42(62-57(67)61-41)36-79-80(58(5,6)7,43-22-14-10-15-23-43)44-24-16-11-17-25-44/h9-17,20-25,37-39,41-42,45-47,51,68H,18-19,26-36H2,1-8H3,(H2,59,69)(H,60,70)(H,61,62)(H,63,73)(H,64,75)(H,65,74)(H,66,72)/p+1/t38-,39+,41-,42-,45-,46-,47-,51-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid
Curated by ChEMBL
| Assay Description
Competitive inhibition against HIV-1 Protease |
J Med Chem 46: 5196-207 (2003)
Article DOI: 10.1021/jm030871u
BindingDB Entry DOI: 10.7270/Q2C53MKZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50135842
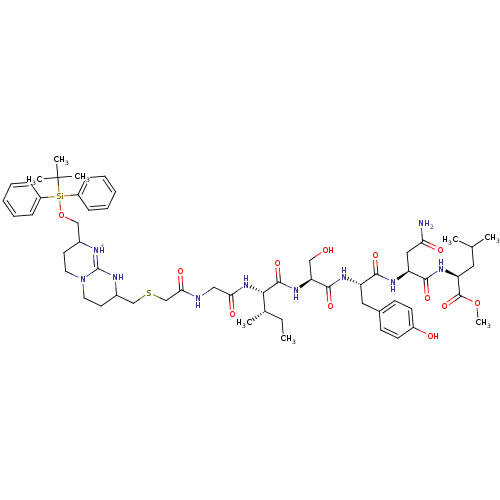
(Bicyclic Guanidinium Subunit | CHEMBL386994)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)CSCC1CCN2CCC(CO[Si](c3ccccc3)(c3ccccc3)C(C)(C)C)[NH+]=C2N1)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)OC |c:43| Show InChI InChI=1S/C58H84N10O12SSi/c1-9-37(4)51(55(77)66-47(32-69)54(76)63-44(29-38-20-22-41(70)23-21-38)52(74)64-45(30-48(59)71)53(75)65-46(28-36(2)3)56(78)79-8)67-49(72)31-60-50(73)35-81-34-40-25-27-68-26-24-39(61-57(68)62-40)33-80-82(58(5,6)7,42-16-12-10-13-17-42)43-18-14-11-15-19-43/h10-23,36-37,39-40,44-47,51,69-70H,9,24-35H2,1-8H3,(H2,59,71)(H,60,73)(H,61,62)(H,63,76)(H,64,74)(H,65,75)(H,66,77)(H,67,72)/p+1/t37-,39?,40?,44-,45-,46-,47-,51-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid
Curated by ChEMBL
| Assay Description
Competitive inhibition against HIV-1 Protease |
J Med Chem 46: 5196-207 (2003)
Article DOI: 10.1021/jm030871u
BindingDB Entry DOI: 10.7270/Q2C53MKZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156914

((S)-methyl 2-((S)-2-((S)-2-(4-(7-(4-((2S,3S)-1-((2...)Show SMILES CC[C@H](C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C45H70N6O11/c1-11-28(8)39(44(58)51-40(29(9)52)41(46)55)49-36(54)15-13-21-62-33-19-17-30-16-18-32(23-31(30)24-33)61-20-12-14-35(53)48-37(26(4)5)43(57)47-34(22-25(2)3)42(56)50-38(27(6)7)45(59)60-10/h16-19,23-29,34,37-40,52H,11-15,20-22H2,1-10H3,(H2,46,55)(H,47,57)(H,48,53)(H,49,54)(H,50,56)(H,51,58)/t28-,29+,34-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50135847
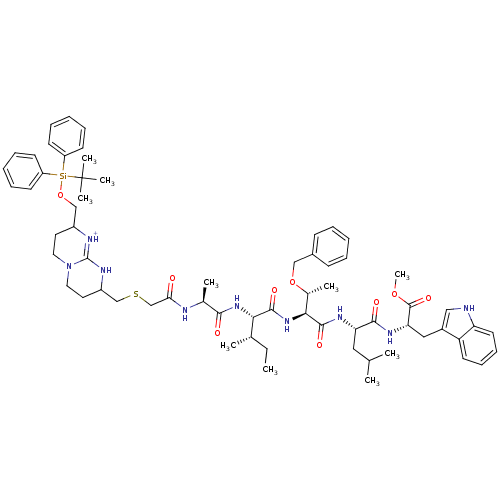
(Bicyclic Guanidinium Subunit | CHEMBL404936)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)CSCC1CCN2CCC(CO[Si](c3ccccc3)(c3ccccc3)C(C)(C)C)[NH+]=C2N1)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OC |c:44| Show InChI InChI=1S/C65H89N9O9SSi/c1-11-43(4)57(61(78)73-58(45(6)82-38-46-23-15-12-16-24-46)62(79)70-54(35-42(2)3)60(77)71-55(63(80)81-10)36-47-37-66-53-30-22-21-29-52(47)53)72-59(76)44(5)67-56(75)41-84-40-49-32-34-74-33-31-48(68-64(74)69-49)39-83-85(65(7,8)9,50-25-17-13-18-26-50)51-27-19-14-20-28-51/h12-30,37,42-45,48-49,54-55,57-58,66H,11,31-36,38-41H2,1-10H3,(H,67,75)(H,68,69)(H,70,79)(H,71,77)(H,72,76)(H,73,78)/p+1/t43-,44-,45+,48?,49?,54-,55-,57-,58-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid
Curated by ChEMBL
| Assay Description
Competitive inhibition against HIV-1 Protease |
J Med Chem 46: 5196-207 (2003)
Article DOI: 10.1021/jm030871u
BindingDB Entry DOI: 10.7270/Q2C53MKZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191465
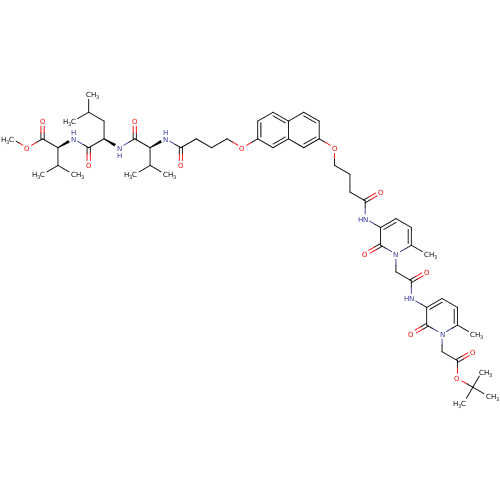
(CHEMBL386205 | methyl (2S)-2-[(2R)-2-[(2S)-2-[4-({...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)Nc3ccc(C)n(CC(=O)Nc4ccc(C)n(CC(=O)OC(C)(C)C)c4=O)c3=O)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C55H75N7O13/c1-32(2)27-43(50(67)60-49(34(5)6)54(71)72-12)58-51(68)48(33(3)4)59-45(64)16-14-26-74-40-22-20-37-19-21-39(28-38(37)29-40)73-25-13-15-44(63)56-41-23-17-35(7)61(52(41)69)30-46(65)57-42-24-18-36(8)62(53(42)70)31-47(66)75-55(9,10)11/h17-24,28-29,32-34,43,48-49H,13-16,25-27,30-31H2,1-12H3,(H,56,63)(H,57,65)(H,58,68)(H,59,64)(H,60,67)/t43-,48+,49+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50191466
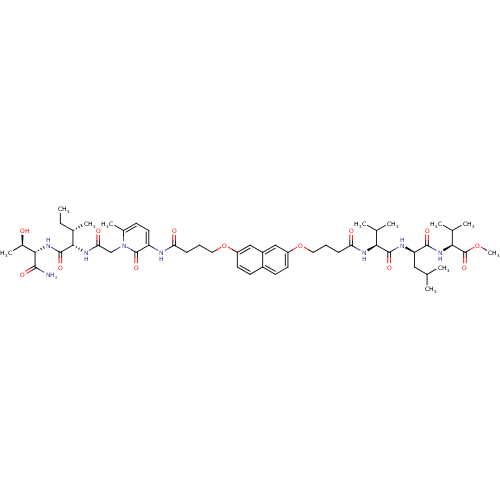
(CHEMBL426149 | methyl (2S)-2-[(2R)-2-[(2S)-2-(4-{[...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cn1c(C)ccc(NC(=O)CCCOc2ccc3ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc3c2)c1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C53H78N8O13/c1-12-32(8)46(51(69)60-47(34(10)62)48(54)66)58-43(65)28-61-33(9)17-22-39(52(61)70)55-41(63)15-13-23-73-37-20-18-35-19-21-38(27-36(35)26-37)74-24-14-16-42(64)57-44(30(4)5)50(68)56-40(25-29(2)3)49(67)59-45(31(6)7)53(71)72-11/h17-22,26-27,29-32,34,40,44-47,62H,12-16,23-25,28H2,1-11H3,(H2,54,66)(H,55,63)(H,56,68)(H,57,64)(H,58,65)(H,59,67)(H,60,69)/t32-,34+,40+,44-,45-,46-,47-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 49: 4657-64 (2006)
Article DOI: 10.1021/jm060576k
BindingDB Entry DOI: 10.7270/Q2KD1XJR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156905

((S)-methyl 2-((2S,3R)-3-hydroxy-2-(4-(7-(4-((S)-1-...)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)[C@@H](C)O |r| Show InChI InChI=1S/C46H71N5O12/c1-26(2)22-35(42(55)51-40(29(7)8)46(59)61-11)47-43(56)39(28(5)6)49-37(53)14-12-20-62-33-18-16-31-17-19-34(25-32(31)24-33)63-21-13-15-38(54)50-41(30(9)52)44(57)48-36(23-27(3)4)45(58)60-10/h16-19,24-30,35-36,39-41,52H,12-15,20-23H2,1-11H3,(H,47,56)(H,48,57)(H,49,53)(H,50,54)(H,51,55)/t30-,35+,36+,39+,40+,41+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50368614
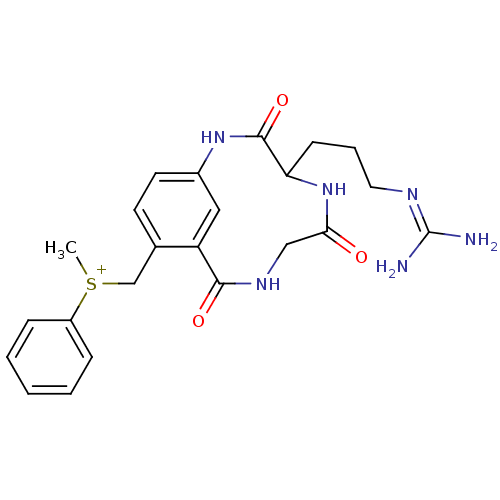
(CHEMBL540392)Show SMILES [#6][S+]([#6]-c1ccc2-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-c1c2)c1ccccc1 Show InChI InChI=1S/C23H28N6O3S/c1-33(17-6-3-2-4-7-17)14-15-9-10-16-12-18(15)21(31)27-13-20(30)29-19(22(32)28-16)8-5-11-26-23(24)25/h2-4,6-7,9-10,12,19H,5,8,11,13-14H2,1H3,(H6-,24,25,26,27,28,29,30,31,32)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against bovine trypsin |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50051760
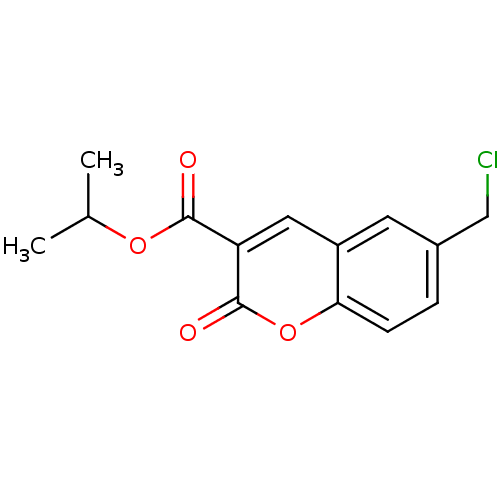
(6-Chloromethyl-2-oxo-2H-chromene-3-carboxylic acid...)Show InChI InChI=1S/C14H13ClO4/c1-8(2)18-13(16)11-6-10-5-9(7-15)3-4-12(10)19-14(11)17/h3-6,8H,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Anionic trypsin
(Bos taurus) | BDBM50047473
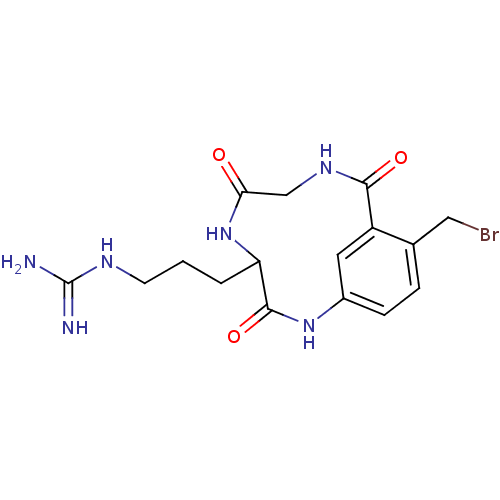
(CHEMBL38512 | N-[3-(11-Bromomethyl-3,6,9-trioxo-2,...)Show SMILES NC(=N)NCCCC1NC(=O)CNC(=O)c2cc(NC1=O)ccc2CBr Show InChI InChI=1S/C16H21BrN6O3/c17-7-9-3-4-10-6-11(9)14(25)21-8-13(24)23-12(15(26)22-10)2-1-5-20-16(18)19/h3-4,6,12H,1-2,5,7-8H2,(H,21,25)(H,22,26)(H,23,24)(H4,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against porcine trypsin |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50051764
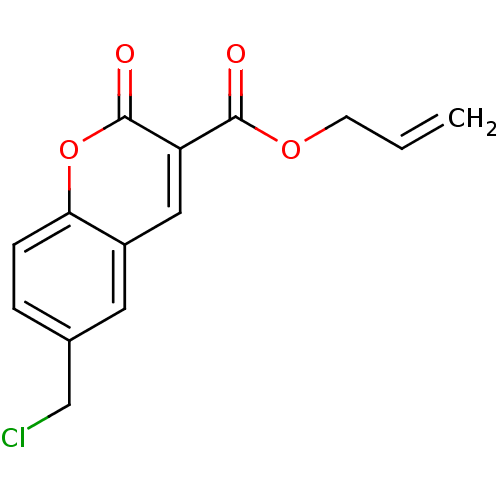
(6-Chloromethyl-2-oxo-2H-chromene-3-carboxylic acid...)Show InChI InChI=1S/C14H11ClO4/c1-2-5-18-13(16)11-7-10-6-9(8-15)3-4-12(10)19-14(11)17/h2-4,6-7H,1,5,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50368615

(CHEMBL553244)Show SMILES [#6][S+]([#6])[#6]-c1ccc2-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-c1c2 Show InChI InChI=1S/C18H26N6O3S/c1-28(2)10-11-5-6-12-8-13(11)16(26)22-9-15(25)24-14(17(27)23-12)4-3-7-21-18(19)20/h5-6,8,14H,3-4,7,9-10H2,1-2H3,(H6-,19,20,21,22,23,24,25,26,27)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against Human urokinase |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50047473

(CHEMBL38512 | N-[3-(11-Bromomethyl-3,6,9-trioxo-2,...)Show SMILES NC(=N)NCCCC1NC(=O)CNC(=O)c2cc(NC1=O)ccc2CBr Show InChI InChI=1S/C16H21BrN6O3/c17-7-9-3-4-10-6-11(9)14(25)21-8-13(24)23-12(15(26)22-10)2-1-5-20-16(18)19/h3-4,6,12H,1-2,5,7-8H2,(H,21,25)(H,22,26)(H,23,24)(H4,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against bovine trypsin |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50051765
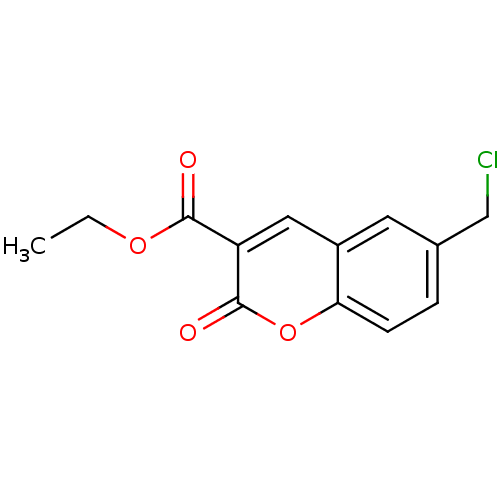
(6-Chloromethyl-2-oxo-2H-chromene-3-carboxylic acid...)Show InChI InChI=1S/C13H11ClO4/c1-2-17-12(15)10-6-9-5-8(7-14)3-4-11(9)18-13(10)16/h3-6H,2,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against alpha-chymotrypsin |
J Med Chem 39: 2579-85 (1996)
Article DOI: 10.1021/jm960090b
BindingDB Entry DOI: 10.7270/Q2QJ7GC1 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50047476
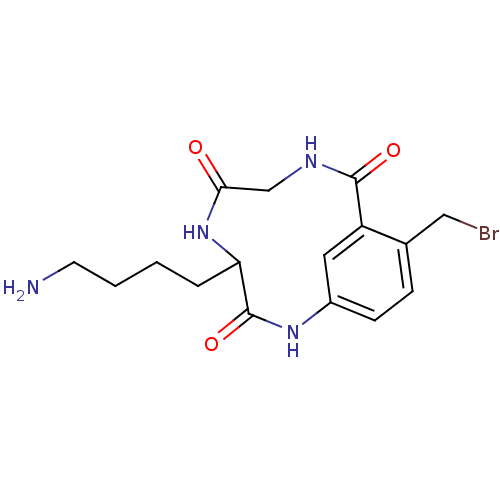
(4-(4-Amino-butyl)-11-bromomethyl-2,5,8-triaza-bicy...)Show InChI InChI=1S/C16H21BrN4O3/c17-8-10-4-5-11-7-12(10)15(23)19-9-14(22)21-13(16(24)20-11)3-1-2-6-18/h4-5,7,13H,1-3,6,8-9,18H2,(H,19,23)(H,20,24)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against bovine trypsin |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50368613
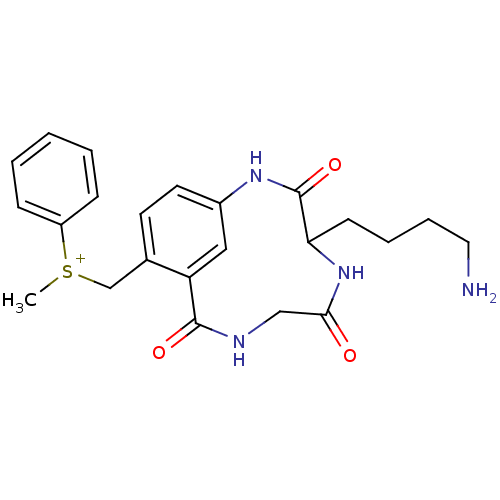
(CHEMBL557221)Show SMILES C[S+](Cc1ccc2NC(=O)C(CCCCN)NC(=O)CNC(=O)c1c2)c1ccccc1 Show InChI InChI=1S/C23H28N4O3S/c1-31(18-7-3-2-4-8-18)15-16-10-11-17-13-19(16)22(29)25-14-21(28)27-20(23(30)26-17)9-5-6-12-24/h2-4,7-8,10-11,13,20H,5-6,9,12,14-15,24H2,1H3,(H2-,25,26,27,28,29,30)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against bovine trypsin |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50368614

(CHEMBL540392)Show SMILES [#6][S+]([#6]-c1ccc2-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-c1c2)c1ccccc1 Show InChI InChI=1S/C23H28N6O3S/c1-33(17-6-3-2-4-7-17)14-15-9-10-16-12-18(15)21(31)27-13-20(30)29-19(22(32)28-16)8-5-11-26-23(24)25/h2-4,6-7,9-10,12,19H,5,8,11,13-14H2,1H3,(H6-,24,25,26,27,28,29,30,31,32)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against Human urokinase |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50047473

(CHEMBL38512 | N-[3-(11-Bromomethyl-3,6,9-trioxo-2,...)Show SMILES NC(=N)NCCCC1NC(=O)CNC(=O)c2cc(NC1=O)ccc2CBr Show InChI InChI=1S/C16H21BrN6O3/c17-7-9-3-4-10-6-11(9)14(25)21-8-13(24)23-12(15(26)22-10)2-1-5-20-16(18)19/h3-4,6,12H,1-2,5,7-8H2,(H,21,25)(H,22,26)(H,23,24)(H4,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against Human urokinase |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50368613

(CHEMBL557221)Show SMILES C[S+](Cc1ccc2NC(=O)C(CCCCN)NC(=O)CNC(=O)c1c2)c1ccccc1 Show InChI InChI=1S/C23H28N4O3S/c1-31(18-7-3-2-4-8-18)15-16-10-11-17-13-19(16)22(29)25-14-21(28)27-20(23(30)26-17)9-5-6-12-24/h2-4,7-8,10-11,13,20H,5-6,9,12,14-15,24H2,1H3,(H2-,25,26,27,28,29,30)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-CERCOA
Curated by ChEMBL
| Assay Description
Apparent binding constant against Human urokinase |
J Med Chem 36: 1539-47 (1993)
BindingDB Entry DOI: 10.7270/Q29C6Z1H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156910

((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)nc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C58H86N8O14/c1-33(2)29-43(52(59)71)63-53(72)41(24-26-49(70)80-58(9,10)11)62-54(73)45(31-37-17-21-39(67)22-18-37)60-46(68)15-14-28-79-48-25-20-38-19-23-40(32-42(38)61-48)78-27-13-16-47(69)65-50(35(5)6)56(75)64-44(30-34(3)4)55(74)66-51(36(7)8)57(76)77-12/h17-23,25,32-36,41,43-45,50-51,67H,13-16,24,26-31H2,1-12H3,(H2,59,71)(H,60,68)(H,62,73)(H,63,72)(H,64,75)(H,65,69)(H,66,74)/t41-,43-,44-,45-,50-,51-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156908

((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](CCC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)cc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C50H78N6O12/c1-29(2)25-38(45(51)60)53-46(61)37(21-22-42(59)68-50(9,10)11)52-40(57)15-13-23-66-35-19-17-33-18-20-36(28-34(33)27-35)67-24-14-16-41(58)55-43(31(5)6)48(63)54-39(26-30(3)4)47(62)56-44(32(7)8)49(64)65-12/h17-20,27-32,37-39,43-44H,13-16,21-26H2,1-12H3,(H2,51,60)(H,52,57)(H,53,61)(H,54,63)(H,55,58)(H,56,62)/t37-,38-,39-,43-,44-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 47: 6392-400 (2004)
Article DOI: 10.1021/jm040833q
BindingDB Entry DOI: 10.7270/Q2CV4H7H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data