 Found 23 hits with Last Name = 'bolger' and Initial = 'g'
Found 23 hits with Last Name = 'bolger' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50326056
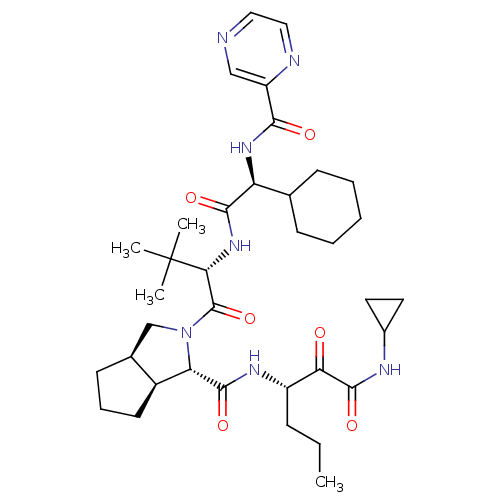
((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)NC1CC1 |r| Show InChI InChI=1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25-,27-,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 60 mins fluorescence assay |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50072317
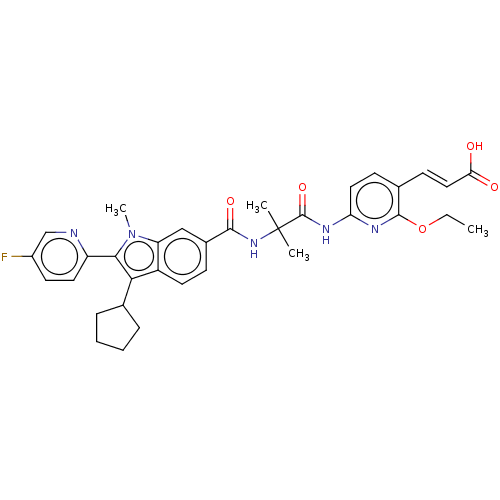
(CHEMBL3408841)Show SMILES CCOc1nc(NC(=O)C(C)(C)NC(=O)c2ccc3c(C4CCCC4)c(-c4ccc(F)cn4)n(C)c3c2)ccc1\C=C\C(O)=O Show InChI InChI=1S/C34H36FN5O5/c1-5-45-32-21(12-17-28(41)42)11-16-27(37-32)38-33(44)34(2,3)39-31(43)22-10-14-24-26(18-22)40(4)30(25-15-13-23(35)19-36-25)29(24)20-8-6-7-9-20/h10-20H,5-9H2,1-4H3,(H,39,43)(H,41,42)(H,37,38,44)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072319

(CHEMBL3408622)Show SMILES CCOc1nc(NC(=O)C2(CCC2)NC(=O)c2ccc3n(C4CCCCC4)c(c(C)c3c2)-c2ccc(F)cn2)ccc1\C=C\C(O)=O Show InChI InChI=1S/C36H38FN5O5/c1-3-47-34-23(12-17-31(43)44)11-16-30(39-34)40-35(46)36(18-7-19-36)41-33(45)24-10-15-29-27(20-24)22(2)32(28-14-13-25(37)21-38-28)42(29)26-8-5-4-6-9-26/h10-17,20-21,26H,3-9,18-19H2,1-2H3,(H,41,45)(H,43,44)(H,39,40,46)/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072318

(CHEMBL3408617)Show SMILES CCOc1nc(NC(=O)C2(CCC2)NC(=O)c2ccc3c(C4CCCC4)c(-c4ccc(F)cn4)n(C)c3c2)ccc1\C=C\C(O)=O Show InChI InChI=1S/C35H36FN5O5/c1-3-46-33-22(11-16-29(42)43)10-15-28(38-33)39-34(45)35(17-6-18-35)40-32(44)23-9-13-25-27(19-23)41(2)31(26-14-12-24(36)20-37-26)30(25)21-7-4-5-8-21/h9-16,19-21H,3-8,17-18H2,1-2H3,(H,40,44)(H,42,43)(H,38,39,45)/b16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50326056

((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)NC1CC1 |r| Show InChI InChI=1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25-,27-,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CatB after 60 mins fluorescence assay |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Rattus norvegicus) | BDBM50336545
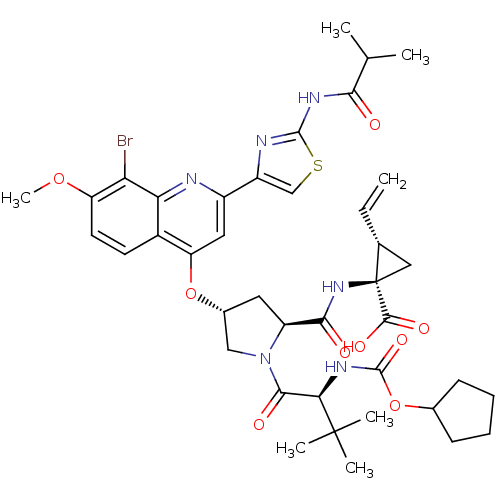
((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(C[C@H]3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21-,23-,27+,32-,40-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat hapatic, acyl coA cholesterol acetyltransferase |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50072320

(CHEMBL3408847)Show SMILES CCOc1nc(NC(=O)C(C)(C)NC(=O)c2ccc3c(C4CCCC4)c(-c4ncc(Cl)cn4)n(C)c3c2)cnc1\C=C\C(O)=O Show InChI InChI=1S/C32H34ClN7O5/c1-5-45-30-22(12-13-25(41)42)34-17-24(37-30)38-31(44)32(2,3)39-29(43)19-10-11-21-23(14-19)40(4)27(26(21)18-8-6-7-9-18)28-35-15-20(33)16-36-28/h10-18H,5-9H2,1-4H3,(H,39,43)(H,41,42)(H,37,38,44)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of serotonin 5-HT2A receptor (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50072317

(CHEMBL3408841)Show SMILES CCOc1nc(NC(=O)C(C)(C)NC(=O)c2ccc3c(C4CCCC4)c(-c4ccc(F)cn4)n(C)c3c2)ccc1\C=C\C(O)=O Show InChI InChI=1S/C34H36FN5O5/c1-5-45-32-21(12-17-28(41)42)11-16-27(37-32)38-33(44)34(2,3)39-31(43)22-10-14-24-26(18-22)40(4)30(25-15-13-23(35)19-36-25)29(24)20-8-6-7-9-20/h10-20H,5-9H2,1-4H3,(H,39,43)(H,41,42)(H,37,38,44)/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of serotonin 5-HT2A receptor (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50072317

(CHEMBL3408841)Show SMILES CCOc1nc(NC(=O)C(C)(C)NC(=O)c2ccc3c(C4CCCC4)c(-c4ccc(F)cn4)n(C)c3c2)ccc1\C=C\C(O)=O Show InChI InChI=1S/C34H36FN5O5/c1-5-45-32-21(12-17-28(41)42)11-16-27(37-32)38-33(44)34(2,3)39-31(43)22-10-14-24-26(18-22)40(4)30(25-15-13-23(35)19-36-25)29(24)20-8-6-7-9-20/h10-20H,5-9H2,1-4H3,(H,39,43)(H,41,42)(H,37,38,44)/b17-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50072317

(CHEMBL3408841)Show SMILES CCOc1nc(NC(=O)C(C)(C)NC(=O)c2ccc3c(C4CCCC4)c(-c4ccc(F)cn4)n(C)c3c2)ccc1\C=C\C(O)=O Show InChI InChI=1S/C34H36FN5O5/c1-5-45-32-21(12-17-28(41)42)11-16-27(37-32)38-33(44)34(2,3)39-31(43)22-10-14-24-26(18-22)40(4)30(25-15-13-23(35)19-36-25)29(24)20-8-6-7-9-20/h10-20H,5-9H2,1-4H3,(H,39,43)(H,41,42)(H,37,38,44)/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM12311
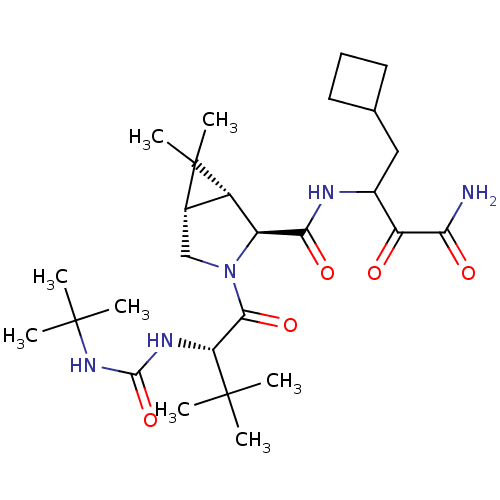
((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...)Show SMILES CC(C)(C)NC(=O)N[C@H](C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)NC(CC1CCC1)C(=O)C(N)=O)C2(C)C)C(C)(C)C |r| Show InChI InChI=1S/C27H45N5O5/c1-25(2,3)20(30-24(37)31-26(4,5)6)23(36)32-13-15-17(27(15,7)8)18(32)22(35)29-16(19(33)21(28)34)12-14-10-9-11-14/h14-18,20H,9-13H2,1-8H3,(H2,28,34)(H,29,35)(H2,30,31,37)/t15-,16?,17-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CatB after 60 mins fluorescence assay |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50336545

((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(C[C@H]3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21-,23-,27+,32-,40-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human PP2B |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of alpha2A adrenergic receptor (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50398052

(CHEMBL2181776)Show SMILES Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)C(=O)Nc1ccc(\C=C\C(O)=O)cc1)-c1ccccn1 Show InChI InChI=1S/C34H34N4O4/c1-38-28-21-24(13-16-26(28)30(23-7-2-3-8-23)31(38)27-9-4-5-20-35-27)32(41)37-34(18-6-19-34)33(42)36-25-14-10-22(11-15-25)12-17-29(39)40/h4-5,9-17,20-21,23H,2-3,6-8,18-19H2,1H3,(H,36,42)(H,37,41)(H,39,40)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A (unknown origin) |
Bioorg Med Chem Lett 25: 1140-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.078
BindingDB Entry DOI: 10.7270/Q2M32XF0 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM12311

((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...)Show SMILES CC(C)(C)NC(=O)N[C@H](C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)NC(CC1CCC1)C(=O)C(N)=O)C2(C)C)C(C)(C)C |r| Show InChI InChI=1S/C27H45N5O5/c1-25(2,3)20(30-24(37)31-26(4,5)6)23(36)32-13-15-17(27(15,7)8)18(32)22(35)29-16(19(33)21(28)34)12-14-10-9-11-14/h14-18,20H,9-13H2,1-8H3,(H2,28,34)(H,29,35)(H2,30,31,37)/t15-,16?,17-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 60 mins fluorescence assay |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50336545

((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(C[C@H]3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21-,23-,27+,32-,40-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CatB after 60 mins fluorescence assay |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50336545

((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(C[C@H]3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21-,23-,27+,32-,40-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 60 mins fluorescence assay |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Mus musculus) | BDBM50336545

((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(C[C@H]3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21-,23-,27+,32-,40-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant cathepsin E by fluorimetry |
Antimicrob Agents Chemother 54: 4611-8 (2010)
Article DOI: 10.1128/AAC.00787-10
BindingDB Entry DOI: 10.7270/Q2NP24Q7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data